The production of one ton of Ordinary Portland Cement releases considerable amounts of CO2 into the atmosphere. As the need and demand for this material grows exponentially, it has become a challenge to increase its production at a time when climate-related problems represent a major global concern. The two main CO2 contributors in this process are fossil fuel combustion to heat the rotary kiln and the chemical reaction associated with the calcination process, in the production of the clinker, the main component of OPC. The current entry presents a critical review of the existent alternative clinker technologies (ACTs) that are under an investigation trial phase or under restricted use for niche applications and that lead to reduced emissions of CO2.
1. Introduction
Ordinary Portland Cement (OPC) had a major impact on the progress of our civilization during the last century
[1]. This cheap mineral binder, when in contact with water, goes through a set of relatively complex physicochemical reactions, that result in a stone-like hard material. This allows the production of mortars, cement mixed with water and sand, and concrete, cement mixed with water, sand and aggregates such as gravel and slag
[2]. Concrete is, not only extremely resilient and durable but can also bear heavy compressive loads and resist severe environmental conditions. These set of properties combined allowed concrete to position as the man-made most widely used material in the world
[3]. Due to the high demand for concrete, 4.3 Gigatons of cement were estimated to be produced globally during the year 2020
[4].However, the amount of CO
2 released during the production of OPC has a very strong environmental impact. In fact, the production of one ton of clinker releases about 0.83 tons of CO
2 and the production of one ton of OPC releases about 0.54 ton of CO
2 [5] making this industry responsible for 5% to 8% of total anthropogenic greenhouse gases
[6] The two main sources of CO
2 emissions resulting from cement production are: (i) the decarbonation of limestone since CaCO
3 is decomposed into CaO and CO
2 at temperatures above 550 °C, with this contribution representing about 60 to 65% of the total CO
2 emissions
[7] and (ii) the fossil fuel combustion to heat the cement kiln, which is responsible for the remaining 35 to 40% of the emissions.
Hence, within the scope of the 2030 United Nations (UN) agenda
[8] and also driven by the increasingly higher CO
2 emission taxes it has become a target and a challenge for the cement industry to develop new binders with a lower ecologic footprint, that can be produced at a large scale, so that it can be used as a commodity, without compromising the technical, economic and workability qualities characteristic of OPC. As it became clear from the 26th UN Climate Change Conference of the Parties (COP26), held in Glasgow in November 2021, managing the pressure for the unavoidable need of social development together with the agendas of environmental sustainability and climate change control will be the challenge of the century, and the cement industry itself is a relevant key player in this fundamental discussion.
Until now, the methods that have been studied to mitigate CO
2 emissions in cement production follow five main approaches
[9]:
-
Reduction of the cement-to-clinker ratio, by replacing clinker with supplementary cementitious materials (SCM’s);
-
The use of alternative fuels in the production of clinker together with the increase of energy efficiency of the kiln process;
-
Carbon capture, use and storage (CCUS), i.e., the sequestration and use of the emitted CO2 for specific applications;
-
Electrification of the clinker production process, especially if renewable electricity produced from non-fossil energy sources is used;
- Development of alternative clinker technologies (ACTs), that lead to lower CO2 emissions.
2. Belite-Rich Clinkers
Belitic binders are not a recent discovery. In fact, they have been used since the times of the Roman Empire
[10].This type of clinkers has essentially belite in its constitution and, therefore, its reaction process requires 10% less limestone, as it results from the analysis of
Figure 1. Also, the synthesis of belitic clinkers requires lower processing temperatures, compared with the alitic-based clinkers, which also translates into a reduction of the CO
2 emissions resulting from the furnace heating
[10]. In addition, its lower heat of hydration
[11], its better rheological properties and its improved durability at later ages, due not only to the smaller proportion of CH that is formed in the hydration but also because of its densely packed structure are advantages of belitic clinkers when compared to alitic ones.
Nevertheless, belite clinkers present a low early-age strength, due to their slower hydraulic kinetics. In fact, Kotsay et al.
[10], reported, that after 28 days, the degree of hydration of the belite can be four times lower compared to the alite phase and, only after one year of hardening the strength of alite and belite hydrates are comparable. There are two main reasons for the lower hydraulic reactivity of belite at early ages: one is that the H
2O molecules have more difficulty in penetrating the belite lattice, due to its densely packed structure
[10], the other is that the Ca
2+ ions attached to the SiO
4 tetrahedron are less easily dissolved
[12]. Therefore, the first step of the hydraulic reaction, the dissolution step, is slower in belite, as compared with alite. It has been disclosed that the incorporation of metal oxides into the lattice of belite, as substitutes of Si, increases the hydraulic reactivity at early ages of belite-rich clinker due to a higher infiltration of H
2O molecules into the lattice, accelerating the dissolution of the material
[13].
A completely different approach to belitic clinkers was proposed some years ago in which hydraulic binders with C/S = 1.4 were produced by inducing the formation of a dendritic belite phase embedded in an amorphous calcium-silicate phase
[14]. These hydraulic binders were produced by a process involving heating the raw materials with a specified C/S ratio to a temperature below the liquid’s surface, followed by a two-step cooling ramp, in order to obtain during solidification a dendritic morphology of the crystalline phase. After milling the clinker obtained by this process, and by adding up to 25% of water, the paste set, showing mechanical performance that went up to four times higher than the values obtained for a reference round shape belite clinker, opening the possibility of developing a novel belite-based clinker with increased reactivity.
Therefore, belitic-rich clinkers can be used in conditions when factors such as low heat release and high later age strength are important parameters, for example in high-performance concrete, or large volume structures
[11]. Nevertheless, although the substitution of alite by belite may reduce CO
2 process-related emissions by up to 10%, it is still far from the goals defined for the cement industry in the global agenda for climate change.
3. Calcium Sulfoaluminate Cements and Belite-Ye’elimite-Ferrite Cements
Calcium sulfoaluminate cements (CSACs) are a belitic type of cement, which were developed in the 1970s
[15] with the intention of compensating for the lower early-age strengths typically observed in belite-rich cements
[15]. Typical raw materials used in the production of CSACs are limestone, calcium sulfate and aluminum-rich minerals or industrial by-products. Its production is carried out at temperatures around 1250 °C, approximately 200 °C lower than the necessary to produce OPC clinker
[15], and is generally easier to grind
[16].
The main clinker phases in CSACs are ye’elimite, Ca
4(AlO
2)
6SO
4, belite and calcium sulfate CaSO
4 [15]. Since ye’elimite rapidly hydrates, it compensates for the loss in early-age strength in belitic clinkers
[17]. As ye’elimite dissolves it enables the reaction with calcium sulfate and water and allows the formation of ettringite (Ca
6Al
2(SO
4)
3(OH)
12·26H
2O) and microcrystalline aluminum hydroxide Al(OH)
2 [16]. Ye’elimite contains about 50% wt. of Al
2O
3 thus, the required alumina content in the raw materials to produce CSACs is above 20%, which can come from sources such as bauxite or industrial by-products, such as the ones proposed in the work by Canbek et al., namely red mud and sulfate-rich/high-lime fly ash
[17]. However, the availability of low-cost sources of alumina-rich raw materials is certainly a limitation for the generalized use of CSACs
[17].
Cement with high ye’elimite contents (>50% wt) can be used in combination with OPC to produce a fast-setting, rapid hardening cement
[16]. CSACs cements with less ye’elimite (25–50% wt) contain significant amounts of belite (30–50% wt) and ferrite (5–20% wt) and can be a sustainable replacement material for OPC
[16]. When compared to OPC, in some areas, the use of CSACs has been shown to have better performance when applied to concrete. They present lower shrinkage, lower cracking and higher resistance to freeze-thaw damage
[16].
Another alternative to the CSACs is belite-ye’elimite-ferrite cement (BYFC), which presents a lower cost than CSACs, achieved by reducing the use of the most expensive aluminum-rich raw materials, resulting in a higher proportion of silicate and ferrite phases
[18]. The ferrite phase, 4CaO·Al
2O
3·Fe
2O
3 has a slower hydration process than Ca
4(AlO
2)
6SO
4, therefore, ye’elimite, anhydrite and gypsum are the first phases to react, followed by ferrite and belite
[19] Since the hydration of ye’elimite is faster than that observed for belite, the increase of compressive strength is similar to OPC
[20].
Both CSACs and BYFCs can be produced in common clinker plants, essentially by changing the raw materials that are used to feed the kiln
[18].This is a major advantage in terms of investment cost since it would allow the production of both types of material within the same facilities without the need for substantial process modifications. However, both CSACs and BYFCs present susceptibility to the carbonation process, caused by the dissolution of the atmospheric CO
2 into the pore paste. This reacts with the hydrated products causing an increasing CO
32− ion concentration. The formation of this anion has severe consequences facilitating the deterioration of ettringite
[16], and raising the acidity of the system leading to the corrosion of steel rebar, used to reinforce concrete
[15].
4. The Solidia Cement Approach
Solidia Cement patented in 2016
[21], is a non-hydraulic binder produced using the same raw materials as OPC, but with a lower amount of CaCO3 and a kiln temperature around 1200 °C, which allows a reduction of the CO
2 emissions by 30%
[21][22]. This binder has an overall C/S molar ratio of ~1 and it is formed essentially by wollastonite/pseudowollastonite, with smaller amounts of rankinite (13% wt), and belite (~3% wt)
[23]. This mixture of calcium silicate phases has the ability to harden by a carbonation process and, consequently, there is no need for water consumption for the reaction to occur
[22].
This cement is produced by feeding the granulated raw material into a natural gas-fired rotary kiln. The calcium silicate compositions created in the rotary kiln emerge in a “clinker” form, that is, in small granules with diameters of approximately 1 to 4 mm. The clinker is then ground to a powder with a mean particle size of approximately 12 μm. To produce concrete, this material is mixed with aggregates, sand and water. The cure of the concrete takes place when the mixture is exposed to a high-concentration gaseous CO
2 environment (60–90%)
[24] which allows the reaction of the binder phases and the production of CaCO
3 and SiO
2.
One of the most interesting characteristics of this binder is precisely the fact that the curing process can capture up to 300 kg of CO
2, per ton of binder
[22] and is only limited by the ability of gaseous CO
2 to diffuse throughout the particles
[22]. To speed up the curing process heat may be applied, these temperatures, if needed, can even be higher than 60 °C since there is no formation of ettringite
[24]. The CaCO
3 that is formed fills the pore space within the concrete, creating a dense microstructure and, the SiO
2 is formed at the outer surface of the reacting cement particle
[22].
Although Solidia Cement does not hydrate, water plays an important role in its forming and curing mechanism. Water contributes to the good flowability of the material and also acts as a permeating agent contributing to the cure development that occurs through a counter diffusion process where water molecules are replaced by CO
2 molecules
[24]. However, since the water is not consumed, 90% of it can be recovered, while the remaining is retained in the cured concrete
[23]. The mechanical properties of the concrete are equivalent to those of OPC, and they are achieved within a shorter curing period
[23]. Another characteristic of this binder is that the carbonation process only releases about 87 kJ/mol of heat during curing which is dissipated through the evaporation process of the water that is used in the concrete preparation
[24].
Even though this is a promising cement, its application and use are limited, since its curing process must be conducted under very controlled CO
2 concentration conditions, which, so far, can only be provided in a ready-mixed concrete plant
[25] impairing to some extent the generalized use of Solidia cement as a substitute of OPC.
5. The Celitement Approach
Celitement
® is a patented hydraulic binder, developed by the Karlsruhe Institute of Technology (KIT) in collaboration with the SCHWENK Zement KG industry
[26]. Its concept is to synthesize and stabilize a short-time precursor of C-S-H to produce a hydraulic binder
[27]. This material is characterized by its low energy demand during its production process, which enables a reduction in CO
2 emissions
[27]. The Celitement production relies on the formation of an intermediate phase that is prior to the development of the C-S-H. This intermediate phase has a similar structure to C-S-H, but a slightly different chemical composition and is referred to as hydraulic Calcium Hydro Silicate (hCHS)
[28].
The production method of Celitement, uses raw materials CaO, in the simplest case, or Ca(OH)
2 and quartz sand
[29]. It requires a calcination stage (around 1000 °C) that is applied only to the CaCO
3-rich raw-material, and hydrothermal processing of the raw mix that takes place in an autoclave at a temperature of 200 °C and at a saturated steam pressure of 12 bar, which facilitates the full electrification of the process. The product that results from the autoclave is stabilized by a strong hydrogen bond which makes it non-hydraulically active
[27]. In a second step, this product goes through a special grinding operation
[27] that enables the destruction of the hydrogen bonds
[29] and, around the cores of the non-reactive co-milled silicates, a new amorphous calcium hydrosilicate (hCHS) is produced
[27][28][29]. The final produced material is mainly amorphous, with highly disordered phases and high specific surface, containing in its composition Q
0 and Q
1 silicate species
[29][30]. After 17–20 h of hCHS hydration, a well-ordered C-S-H phase is formed, releasing a very low heat of hydration (120–150 J/g) and promoting an early-age strength comparable to OPC
[27].
The Celitement approach is based on a technology that completely differs from the one known today for the production of Portland cement, leading to what may be a significant drawback in its industrial implementation. Nevertheless, this new technology is already under the demonstration phase, with a recent expansion to the pilot plant, constructed in 2011, allowing the production of approximately 700 kg per day
[31].
6. The C/S≈1 Amorphous Approach (X-Clinker)
Another alternative, developed and patented internationally by CIMPOR and Técnico-Lisbon, is an amorphous low-calcium hydraulic binder characterized by a raw mix containing 33% less CaCO
3 than the typical OPC, and an overall C/S ratio of 1, allowing for a reduction of more than 25% of the usual OPC clinker process-related CO
2 emissions
[32][33].
The production process of this binder allows the use of traditional raw materials, such as limestone, clay, marl, sand, etc., and consists in fully melting the raw mixture, at a temperature of 1550 °C, followed by a rapid cooling
[31]. The resultant product is mostly amorphous (~94% wt), with the presence of a small amount (<10%) of pseudo-wollastonite
[32]. It should be pointed out that the full melting of the mixture may facilitate the electrification of the process through plasma or electrical arc melting, which may lead to a scenario where the effluent gas stream is solely fed by the CO
2 generated by the calcination of the raw meal. Such a highly CO
2 concentrated gas stream could potentially be combined with green H
2 to produce methanol and other hydrocarbons
[34][35].
The reactivity of this novel binder comes, mostly, from its amorphous phase, yet even though existing in a small amount, the presence of pseudo-wollastonite has been shown to have some influence on the hydrated product performance
[36]. Better compressive strength results were obtained when this phase was produced in an amount of ~6% wt
[36]. An investigation on the hydration of this binder observed, by
29SiMAS-NMR spectroscopy, that the least coordinated Q
n units, Q
0 and Q
1, play a very important role in the hydration since they appear to be very prone to polymerize and convert into C-S-H structures that are similar to tobermorite
[32]. By changing the structure from crystalline to amorphous, the arrangements of Si–O bonds become more disordered, which favors their dissolution
[32] and, consequently, the further precipitation of equilibrium hydration products
[37].
The behavior of this novel amorphous binder was further studied by Santos et al.
[38], which investigated the effect of different alkaline activators (Na
2CO
3 and a mixture of NaOH and Na
2SiO
3) on the mechanical strength and structural characteristics of hydrated pastes. It was observed that, when activated, those pastes presented increased hydration kinetics, allowing for an improvement in their mechanical performances. Furthermore, the most competitive results were obtained when the pastes were activated with Na
2SiO
3, with a 3 wt% total content of Na
2O, obtaining pastes with strengths comparable with those of traditional OPC
[38].
In terms of technological development, this approach presents a main drawback, which is the need for a pyro-processing step that is 100 °C superior to that of OPC and, the requirement of a sodium silicate solution for activation, in order to present competitive early-age strength.
In addition, since the processing conditions require the formation of 100% liquid phase, some adaptations to the usual BAT of clinker production may be required in order to industrially implement this type of technology.
7. Summary of the Alternative Clinker Technologies
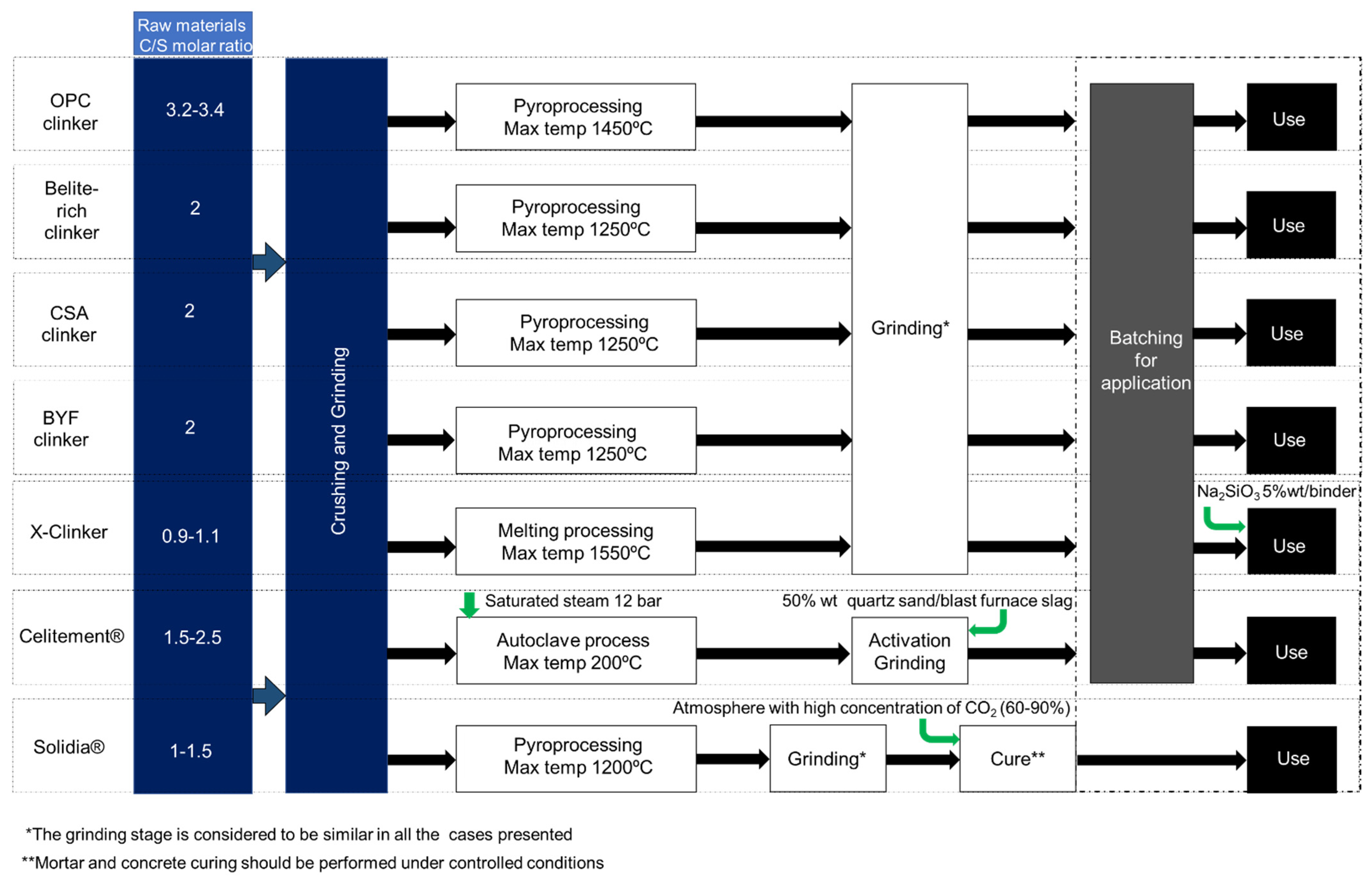
Figure 1 shows a simplified flow chart containing the stages considered for the production of the alternative binders, evidencing the differences of the process in the various ACTs approaches. From Figure 1, it becomes clear that in the present state, research should be pursued in all the presented solutions. From the several technological proposals contained in Figure 1, it should be noted that only the Celitement® and the X-Clinker approaches presently consider a scenario of full process electrification, while the other approaches essentially follow a fuel combustion-based design, similar to the existing BAT. The proximity to the BAT for clinker production is certainly an advantage for the industrial implementation of some of the alternative binders mentioned above, however, when looking forward through a perspective of cement industry decarbonization, it is the author’s belief that full or partial conversion of the existing fuel-based technology to electricity-based technology should occur in the next decades.
Figure 1. Simplified schematic representation of the stages considered within the production processes of the alternative binders reviewed.
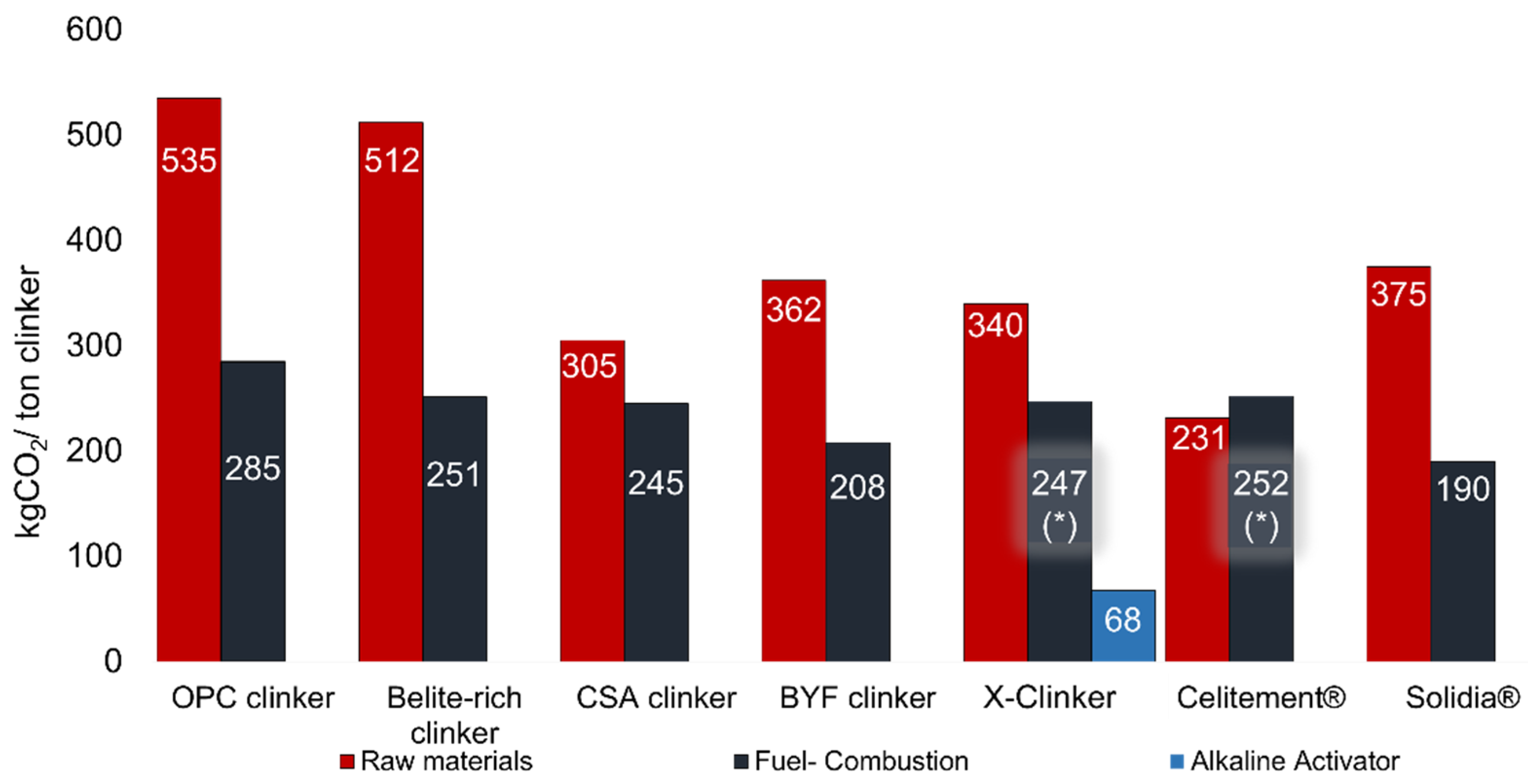
Figure 2 shows a detailed description of the various sources of CO2 emissions within the production processes considered for the ACTs reviewed, evidencing the separation of the contributions for the thermal and material-related emissions. The CSA, X-Clinker and Celitement approaches lead to material-related CO2 emissions smaller than 0.35 tons per ton of clinker. If full electrification of the process for these ACTs with green electricity is achieved, a target for CO2 emission in cement production smaller than 0.25 tons of CO2 per ton of cement is within reach of the cement industry in the upcoming years.
Figure 2. CO2 contributions for the energy- and material-related emissions of the various alternative binders reviewed. NOTE: (*) indicates the technologies that already consider a fully electrified production process and therefore, depending on the nature of the electricity used, the thermal component of CO2 emissions can be completely eliminated.