
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | David Mellor | + 6109 word(s) | 6109 | 2021-12-02 05:08:36 | | | |
| 2 | Vivi Li | -5 word(s) | 6104 | 2022-01-19 04:42:51 | | |
Video Upload Options
Mouth pain in horses, specifically that caused by bits, is evaluated as a significant welfare issue. The conscious experiences of pain generated within the body generally, its roles, and its assessment using behaviour, as well as the sensory functionality of the horse’s mouth, are outlined as background to a more detailed evaluation of mouth pain. Bit-induced mouth pain elicited by compression, laceration, inflammation, impeded blood flow, and the stretching of tissues is considered. Observable signs of mouth pain are behaviours that are present in bitted horses and absent or much less prevalent when they are bit-free. It is noted that many equestrians do not recognise that these behaviours indicate mouth pain, so that the magnitude of the problem is often underestimated.
1. Mouth Pain in Horses
1.1. Bit-Induced Nociceptor Stimulation and Pain
1.1.1. The Interdental Space
- (1).
-
The established relationship between tension (T, units N), mass (m, units kg) and gravitational acceleration (g = 9.8 metres/sec2), which is “T = mg” or “T = 9.8 m” [9].
- (2).
- (3).
-
An estimated area of bit–gum contact on the interdental space (CAbg) of 0.387 cm2 [16], which is equivalent to a 6.22 × 6.22 mm square.
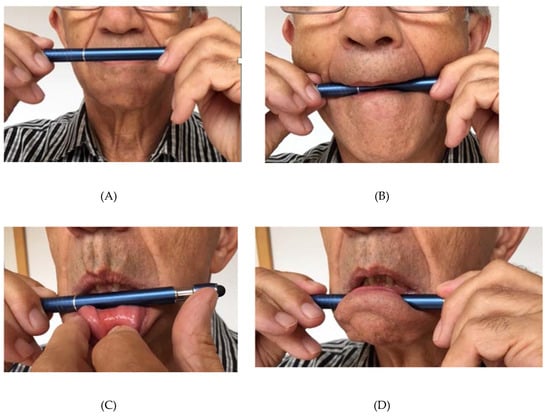
1.1.2. The Tongue
1.1.3. The Commissures of the Lips and the Buccal Mucosa
1.2. General Comments and Summing up
2. Behavioural Indices of Mouth Pain in Horses
| Indicative Pain-Related Behaviours in Ridden Bitted Horses |
| Mouth: resists bridling; fussing with the bit, persistent jaw movements, chewing; crossing the jaw; slightly open or gaping mouth; teeth grinding, holding the bit between the teeth; tongue persistently moving or protruding from the mouth, tongue placed above the bit or retracted behind it; excessive salivation or drooling. Head-neck: sudden evasive movements due to abrupt increases in rein tension; side-to-side or up-down head shaking, jawline above horizontal; head tilted, stiff necked; rein-induced low jowl-angle, neck arched, nasal plane at or behind the vertical; reaches forward so rider uses longer rein. Pain face: identifiable nostril flare, lip positions, ear positions, eye white visibility and facial muscle tension. Body movement/gait: stiff or choppy stride, hair trigger responses, crabbing; difficult to control, hesitant to move forward, difficult to stop, side-stepping from straight-line motion; bucking; rearing; tail swishing. Refs: [52][53][54][55][43][44][15][34][41][45][56][57][58][59][60][61][62][63][64][65][66][67][68]; plus YouTube archive videos a |
| Bitted to Bit-Free Changes in Ridden Horse Behaviour |
| Mouth: all bit-related mouth behaviours absent; quiet, closed mouth, tongue inside mouth and appropriately placed; little or no teeth grinding; no drooling. Head-neck: head shaking absent; lower head-neck position and wider jowl angle; head, neck and spinal column properly aligned longitudinally. Pain face: no indications of mouth-related pain in healthy animals. Body movement/gait: calm, relaxed and cooperative demeanour; engaged, lively, energised and exhibits vitality of fitness; head freedom supports balanced, aligned and smooth rhythm of motion; tail movement in synchrony with spinal movement. Refs: [42][69][70][7][57][58][59][60][61]; plus YouTube archive videos a |
| Behaviours of Bit-Free Horses at Rest or When Running Free |
| As expected, domesticated horses wearing loosely-but-snugly fitted bit-free bridles do not display any of the bit-related behaviours noted above while standing at rest or engaging in exercise ranging from walking to galloping; nor do horses wearing halters while standing in stalls or moving freely in turnout paddocks. Likewise, neither do wild, free-roaming horses when standing alert or when walking, trotting, cantering and galloping during roundups. Refs: [71][72]; YouTube archive videos of bit-free domesticated horses, and of ~150 free-roaming, wild Brumbies (Australia), Camargue horses (France), Kaimanawa horses (New Zealand) and Mustangs (USA) a |
3. Welfare Implications of Bit-Induced Mouth Pain in Horses
3.1. Respiratory Consequences of an Open Mouth and Relocation of the Tongue above or behind the Bit
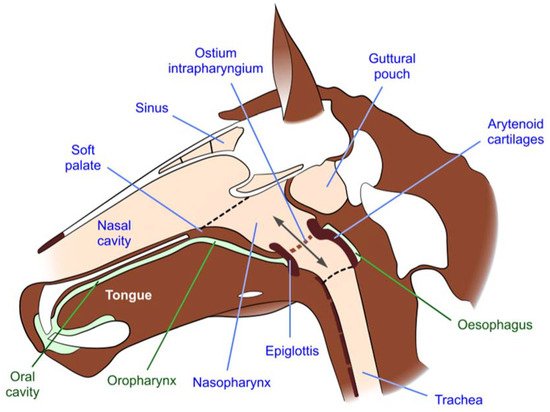
3.2. Respiratory Impacts of Low Jowl Angles Maintained by the Firm Application of Rein Tension
3.3. Pain-Related Conflict Behaviours and Summing up
References
- Haggard, P.; de Boer, L. Oral somatosensory awareness. Neurosci. Biobehav. Rev. 2014, 47, 469–484.
- Cook, W.R. Damage by the bit to the equine interdental space and second lower premolar. Equine Vet. Educ. 2011, 23, 355–360.
- Bennett, D.G. Bits and bitting: Form and function. In Proceedings of the 47th Annual Convention of the American Association of Equine Practitioners, San Diego, CA, USA, 24–28 November 2001; pp. 130–141. Available online: https://pdfs.semanticscholar.org/7a26/a4617e7587d72ffcc2435dd7071d3ad3b6b1.pdf (accessed on 17 February 2020).
- Manfredi, J.; Clayton, H.M.; Rosenstein, D. Radiographic study of bit position within the horse’s oral cavity. Equine Comp. Exerc. Physiol. 2005, 2, 195–201.
- Benoist, C.C.; Cross, G.H. A photographic methodology for analyzing bit position under rein tension. J. Equine Vet. Sci. 2018, 67, 102–111.
- Mantyh, P.W. The neurobiology of skeletal pain. Eur. J. Neurosci. 2014, 39, 508–519.
- Cook, W.R.; Kibler, M. Behavioural assessment of pain in 66 horses, with and without a bit. Equine Vet. Educ. 2019, 31, 551–560.
- Van Lancker, S.; van den Broeck, W.; Simoens, P. Incidence and morphology of bone irregularities of the equine interdental space (bars of the mouth). Equine Vet. Educ. 2007, 19, 103–106.
- College Physics. Tension. In Dynamics: Force and Newton’s Laws of Motion; OpenStax College, Rice University: Huston, Tx, USA; Available online: https://opentextbc.ca/physicstestbook2/chapter/normal-tension-and-other-examples-of-forces/ (accessed on 18 February 2020).
- Clayton, H.M.; Singleton, W.H.; Lanovaz, J.; Cloud, G.L. Measurement of rein tension during horseback riding using strain gage transducers. Exp. Tech. 2003, 27, 34–36.
- Clayton, H.M.; Larson, B.; Kaiser, L.A.J.; Lavagnino, M. Length and elasticity of side reins affect rein tension at trot. Vet. J. 2011, 188, 291–294.
- Egenvall, A.; Eisersiö, M.; Rhodin, M.; van Weeren, R.; Roepstorff, L. Rein tension during canter. Comp. Exerc. Physiol. 2015, 11, 107–117.
- Egenvall, A.; Roepstorff, L.; Eisersiö, M.; Rhodin, M.; van Weeren, R. Stride-related rein tension patterns in walk and trot in the ridden horse. Acta Vet. Scand. 2015, 57, 89.
- Egenvall, A.; Clayton, H.M.; Eisersiö, M.; Roepstorff, L.; Byström, A. Rein tensions in transitions and halts during equestrian dressage training. Animals 2019, 9, 712.
- Piccolo, L.; Kienapfel, K. Voluntary rein tension in horses when moving unridden in s dressage frame compared with ridden tests in the same horses—A pilot study. Animals 2019, 9, 321.
- Cook, W.R.; Strasser, H. Harmful effects of the bit. In Metal in the Mouth: The Abusive Effects of Bitted Bridles; Kells, S., Ed.; Sabine Kells: Qualicum Beach, BC, Canada, 2003; pp. 3–13.
- Heleski, C.R.; McGreevy, P.D.; Kaiser, L.J.; Lavagnino, M.; Tans, E.; Bello, N.; Clayton, H.M. Effects on behaviour and rein tension on horses ridden with or without martingales and rein inserts. Vet. J. 2009, 181, 56–62.
- Christensen, J.W.; Zharkikh, T.L.; Antoine, A.; Malmkvist, J. Rein tension acceptance in young horses in a voluntary test situation. Equine Vet. J. 2011, 43, 223–228.
- Mellor, D.J. Equine Welfare during Exercise: Do We Have a ‘Bit’ of a Problem. PowerPoint Slides Presented at a Professional Development Event, Entitled Sport Horse Welfare and Social Licence to Operate, Mounted by Horse South Australia on 13 and 14 February 2018 at Hahndorf, South Australia. Available online: https://www.slideshare.net/SAHorse/equine-welfare-during-exercise-do-we-have-a-bit-of-a-problem (accessed on 9 February 2020).
- Bendrey, R. New methods for the identification of evidence for bitting on horse remains from archaeological sites. J. Archaeol. Sci. 2007, 34, 1036–1050.
- Mata, F.; Johnson, C.; Bishop, C. A cross-sectional epidemiological study of prevalence and severity of bit-induced oral trauma in polo ponies and race horses. J. Appl. Anim. Welf. Sci. 2015, 18, 259–268.
- Tremaine, W.H. Management of equine mandibular injuries. Equine Vet. Educ. 1998, 10, 146–154.
- Johnson, T.J. Surgical removal of mandibular periostitis bone spurs caused by bit damage. Proc. Am. Assoc. Equine Pract. 2002, 48, 458–462.
- Johnson, J.; Porter, M. Dental conditions affecting the mature performance horse (5–15 years). Proc. Am. Assoc. Equine Pract. 2006, 50, 31–36.
- Tuomola, K.; Maki-Kinnia, N.; Kujala-Wirth, M.; Mykkänen, A.; Valros, A. Oral lesions in the bit area in finnish trotters after a race: Lesion evaluation, scoring and occurrence. Front. Vet. Sci. 2019, 6, 206. Available online: https://doi.org/10.3389/fvets.2019.00206 (accessed on 9 February 2020).
- Björnsdóttir, S.; Frey, R.; Kristjansson, T.; Lundström, T. Bit-related lesions in Icelandic competition horses. Acta Vet. Scand. 2014, 56, 40. Available online: http://www.actavetscand.com/content/56/1/40 (accessed on 9 February 2020).
- Byers, M.R.; Närhi, M.V. Dental injury models: Experimental tools for understanding neuroinflammatory interactions and polymodal nociceptor functions. Crit. Rev. Oral Biol. Med. 1999, 10, 4–39.
- Equus 2019. Tongue Injuries: Wounds to Your Horse’s Tongue Can Easily Go Unnoticed—But That Doesn’t Mean They Can be Ignored. Available online: https://equusmagazine.com/horse-care/tongue-injuries-12258 (accessed on 9 February 2020).
- Pigg, M.; Svensson, P.; List, T. Orofacial thermal thresholds: Time-dependent variability and influence of spatial summation and test site. J. Orofac. Pain 2011, 25, 39–48.
- Tell, A.; Egenvall, A.; Lundstrom, T.; Wattle, O. The prevalence of oral ulceration in Swedish horses when ridden with bit and bridle and when unridden. Vet. J. 2008, 178, 405–410.
- Uldahl, M.; Clayton, H. Lesions associated with the use of bits, nosebands, spurs and whips in Danish competition horses. Equine Vet. Educ. 2018, 51, 154–162.
- Rezaian, M. Absence of hyaline cartilage in the tongue of ‘Caspian miniature horse’. Anat. Histol. Embryol. 2006, 35, 241–246.
- Engelke, E.; Gasse, H. An anatomical study of the rostral part of the equine oral cavity with respect to position and size of a snaffle bit. Equine Vet. Educ. 2003, 15, 158–163.
- Manfredi, J.M.; Rosenstein, D.; Lanovaz, J.L.; Nauwelaerts, S.; Clayton, H.M. Fluoroscopic study of oral behaviours in response to the presence of a bit and the effects of rein tension. Comp. Exerc. Physiol. 2010, 6, 143–148.
- Findley, J.A.; Sealy, H.; Franklin, S.H. Factors associated with tongue tie use in Australian Standardbred racehorses. Equine Vet. J. 2016, 48 (Suppl. 50), 18–19.
- Barakzai, S.Z.; Finnegan, C.; Dixon, P.M.; Hillyer, M.H.; Boden, L.A. Use of tongue ties in thoroughbred racehorses in the United Kingdom, and its association with surgery for dorsal displacement of the soft palate. Vet. Rec. 2009, 165, 278–281.
- Porter, D.; Caraguel, C.; Noschka, E.; Samantha Franklin, S. Tongue-tie use in Australian Thoroughbred horses over a 5-year period (2009–2013). In Proceedings of the World Equine Airway Symposium, Copenhagen, Denmark, 13–15 July 2017; p. 155.
- Franklin, S.H.; Naylor, J.R.; Lane, J.G. The effect of a tongue-tie in horses with dorsal displacement of the soft palate. Equine Vet. J. 2002, 34, 430–433.
- Vandermark, S.; Wilkins, C. Tongue Ties: Trying to See the Whole Picture. Horses and People. August 2019. Available online: https://horsesandpeople.com.au/tongue-ties-trying-to-see-the-whole-picture/ (accessed on 6 March 2020).
- Franklin, S.; McGreevy, P. Over 20% of Australian Horses Race with Their Tongues Tied to Their Lower Jaw. The Conversation. July 2018. Available online: https://theconversation.com/over-20-of-australian-horses-race-with-their-tongues-tied-to-their-lower-jaw-99584 (accessed on 12 February 2020).
- Marsh, L.; McGreevy, P.; Hazel, S.; Santos, L.; Herbart, M.; Franklin, S. The effect of tongue-tie application on stress responses in resting horses. BioRxiv 2019, 634717. Available online: https://www.biorxiv.org/content/10.1101/634717v1.full (accessed on 24 February 2020).
- Cook, W.R. Bit-induced pain: A cause of fear, flight, and facial neuralgia in the horse. Pferdeheilkunde 2003, 19, 75–82.
- Dyson, S.; Berger, J.M.; Ellis, A.D.; Mullard, J. Can the presence of musculoskeletal pain be determined from the facial expressions of ridden horses (FEReq)? J. Vet. Behav. 2017, 19, 78–89.
- Mullard, J.; Berger, J.M.; Ellis, A.D.; Dyson, S. Development of an ethogram to describe facial expressions in ridden horses (FEReq). J. Vet. Behav. 2017, 18, 7–12.
- Dyson, S.; Berger, J.M.; Ellis, A.D.; Mullard, J. Development of an ethogram for a pain scoring system in ridden horses and its application to determine the presence of musculoskeletal pain. J. Vet. Behav. 2018, 23, 47–57.
- Gregory, N.G. Physiology and Behaviour of Animal Suffering; Blackwell Science: Oxford, UK, 2004.
- Muir, W. Recognizing and Treating Pain in Horses, Veterian Key 2016. Available online: https://veteriankey.com/recognizing-and-treating-pain-in-horses/ (accessed on 17 February 2020).
- Hannah, C. The Truth about Bits: Facial Neuralgia. Horse and Human 2009. Available online: http://www.horseandhuman.co.nz/articles/html/the_truth_about_bits_part4.html (accessed on 18 February 2020).
- Mair, T.; Lane, G. Head shaking in horses. In Practice 1990, 12, 183–186.
- Newton, S.A.; Knottenbelt, D.C.; Eldridge, P.R. Headshaking in horses: Possible aetiopathogenesis suggested by the results of diagnostic tests and several treatment regimes used in 20 cases. Equine Vet. J. 2000, 32, 208–216.
- Roberts, V. Trigeminal-mediated headshaking in horses: Prevalence, impact, and management strategies. Vet. Med. Res. Rep. 2019, 10, 1–8.
- Williams, L.R.; Warren-Smith, A.K. Conflict responses exhibited by dressage horses during competition. J. Vet. Behav. Clin. Appl. Res. 2010, 5, 215.
- Waran, N.; Randle, H. What we can measure, we can manage: The importance of using robust welfare indicators in equitation science. Appl. Anim. Behav. Sci. 2017, 190, 74–81.
- Ashley, F.H.; Waterman-Pearson, A.E.; Whay, H.R. Behavioural assessment of pain in horses and donkeys: Application to clinical practice and future studies. Equine Vet. J. 2005, 37, 565–575.
- Dalla Costa, E.; Minero, M.; Lebelt, D.; Stucke, D.; Canali, E.; Leach, M.C. Development of the Horse Grimace Scale (HGS) as a pain assessment tool in horses undergoing routine castration. PLoS ONE 2014, 9, e92281. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3960217/ (accessed on 21 January 2020).
- Cook, W.R. Pathophysiology of bit control in the horse. J. Equine Vet. Sci. 1999, 19, 196–204.
- Cook, W.R.; Mills, D.S. Preliminary study of jointed snaffle vs. crossunder bitless bridles: Quantified comparison of behaviour in four horses. Equine Vet. J. 2009, 41, 827–830.
- Hanson, F.; Cook, R. The Bedouin bridle rediscovered: A welfare, safety and performance enhancer. Horse’s Hoof 2015, 60, 1–8. Available online: http://www.bitlessbridle.com/THEBEDOUBRIDLE.pdf (accessed on 24 February 2020).
- Carey, C. The impact of bitless vs. bitted bridles on the therapeutic riding horse research project. Presented at the Horses in Education and Therapy International Conference on Therapeutic Riding, New Taipei City, Taiwan, 22–25 June 2015; Available online: https://festinalente.ie/equine-assisted-programmes/research/impact-bitless-vs-bitted-bridles-therapeutic-riding-horse/ (accessed on 23 February 2020).
- Carey, C.; Hayes-Moriarty, S.; Brennan, R. The impact of bitted and bitless bridles on the therapeutic riding horse. In Proceedings of the 12th International Equitation Science Conference on Understanding Horses to Improve Training and Performance, Wagga Wagga, Australia, 22–25 November 2016; p. 99. Available online: https://festinalente.ie/wp-content/uploads/2017/04/Impact-of-Bitted-vs-Bitless-Bridles-for-Therapeutic-Riding-Equines.pdf (accessed on 23 February 2020).
- Carey, C.; Brennan, R.; Hayes-Moriarty, S. Further Study on the Impact of Bitted vs. Bitless Bridles for Therapeutic Riding Equines. Festiina Lente Newsletter. January 2020. Available online: https://festinalente.ie/equine-assisted-programmes/research/study-impact-bitted-vs-bitless-bridles-therapeutic-riding-equines/ (accessed on 23 February 2020).
- Polito, R.; Minero, M.; Canali, E.; Verga, M. A pilot study on Yearlings’ reactions to handling in relation to the training method. Anthrozöos 2007, 20, 295–303.
- Visser, E.K.; Van Dierendonck, M.; Ellis, A.D.; Rijksen, C.; Van Reenen, C.G. A comparison of sympathetic and conventional training methods on responses to initial horse training. Vet. J. 2009, 181, 48–52.
- Von Borstel, U.U.; Duncan, I.J.H.; Shoveller, A.K.; Merkies, K.; Linda Jane Keeling, L.J.; Millman, S.T. Impact of riding in a coercively obtained Rollkur posture on welfare and fear of performance horses. Appl. Anim. Behav. Sci. 2009, 228–236.
- McLean, A.N.; McGreevy, P.D. Horse-training techniques that may defy the principles of learning theory and compromise welfare. J. Vet. Behav. 2010, 5, 187–195.
- Hall, C.; Kay, R.; Yarnell, K. Assessing ridden horse behavior: Professional judgment and physiological measures. J. Vet. Behav. 2014, 9, 22–29.
- Górecka-Bruzda, A.; Kosinska, I.; Jaworski, Z.; Tadeusz Jezierski, T.; Murphy, J. Conflict behavior in elite show jumping and dressage horses. J. Vet. Behav. 2015, 10, 137–146.
- Clayton, H. Are Horses Stressed When Bitted for the First Time? Eurodressage. 2019. Available online: http://www.eurodressage.com/2019/01/03/are-horses-stressed-when-bitted-first-time (accessed on 24 February 2020).
- Quick, J.S.; Warren-Smith, A.K. Preliminary investigation of horses’ (Equus caballus) responses to different bridles during foundation training. J. Vet. Behav. 2009, 4, 169–176.
- Jahiel, J. Increase Comfort, Reduce Risk: The Bitless Bridle. Equestrian Medical Safety Association 2014, Fall Newsletter. pp. 5–12. Available online: http://emsaonline.net/wp-content/uploads/gravity_forms/1-5f7def (accessed on 17 February 2020).
- Fraser, A.F. The Behaviour of the Horse; CAB International: Wallingford, UK, 1992.
- Ransom, J.I.; Cade, B.S. Quantifying equid behavior: A research ethogram for free-roaming feral horses. In U.S. Geological Survey Techniques and Methods Report 2-A9; USGS: Reston, VA, USA, 2009.
- Mellor, D.J.; Beausoleil, N.J. Equine welfare during exercise: An evaluation of breathing, breathlessness and bridles. Animals 2017, 7, 41.
- King, M. Bitless: A New Breed of Bridle. The Horse, August 2007. Available online: https://thehorse.com/124806/bitless-a-new-breed-of-bridle/ (accessed on 24 February 2020).
- Hanson, F. A positive reinforcement rein: Rule-changer and game-changer for horsemanship? Horse’s Hoof 2019, 76, 1–14. Available online: https://www.horsetalk.co.nz/2019/10/22/positive-reinforcement-rein-game-changer-horsemanship/ (accessed on 29 February 2020).
- Ambrosiano, N. All about Bitless Bridles for Your Horse: Bit-Free Headgear Is Sometimes the Answer for Sensitive Horses or Tough Training Problems. Equus. 2017. Available online: https://equusmagazine.com/riding/bitless-bridles-092206-10523 (accessed on 24 February 2020).
- Bitless Bridle. Wikipedia. 2019. Available online: https://en.wikipedia.org/wiki/Bitless_bridle (accessed on 24 February 2020).
- Bosal, Wikipedia 2018. Available online: https://en.wikipedia.org/wiki/Bosal (accessed on 24 February 2020).
- Hackamore, Wikipedia 2020. Available online: https://en.wikipedia.org/wiki/Hackamore (accessed on 24 February 2020).
- Ramey, D.W. A historical survey of human-equine interactions. In Equine Welfare; McIlwraith, C.W., Rollin, B.E., Eds.; Wiley-Blackwell: Chichester, UK, 2011; pp. 22–58.
- Whay, H.R.; Main, D.C.J.; Green, L.E.; Webster, A.J.F. Farmer perception of lameness prevalence. In Proceedings of the 12th International Symposium on Lameness in Ruminants, Orlando, FL, USA, 9–13 January 2002; pp. 355–358.
- Barker, Z.E.; Leach, K.A.; Whay, H.R.; Bell, N.J.; Main, D.C.J. Assessment of lameness prevalence and associated risk factors in dairy herds in England and Wales. J. Dairy Sci. 2010, 93, 932–941.
- Horseman, S.V.; Roe, E.J.; Huxley, J.N.; Bell, N.J.; Mason, C.S.; Whay, H.R. The use of in-depth interviews to understand the process of treating lame dairy cows from the farmers’ perspective. Anim. Welf. 2014, 23, 157–165.
- Beausoleil, N.J.; Mellor, D.J. Introducing breathlessness as an animal welfare issue. N. Z. Vet. J. 2015, 63, 44–51.
- Poiseuille’s Law: IV Fluids, Open Anaesthesia. 2017. Available online: https://www.openanesthesia.org/poiseuilles_law_iv_fluids/ (accessed on 7 March 2020).
- Cook, W.R. A hypothetical, aetiological relationship between the horse’s bit, nasopharyngeal oedema and negative pressure pulmonary oedema. Equine Vet. Educ. 2014, 26, 381–389.
- Cook, W.R. Hypothesis article: Bit-induced asphyxia in the racehorse as a cause of sudden death. Equine Vet. J. 2016, 28, 405–409.
- Dixon, P.M.; Railton, D.I.; McGorum, B.C. Temporary bilateral laryngeal paralysis in a horse associated with general anaesthesia and post anaesthetic myositis. Vet. Rec. 1993, 132, 29–32.
- Kollias-Baker, C.A.; Pipers, F.S.; Heard, D.; Seeherman, H. Pulmonary edema associated with transient airway obstruction in three horses. J. Am. Vet. Med Assoc. 1993, 202, 1116–1118.
- Tute, A.S.; Wilkins, P.A.; Gleed, R.D.; Credille, K.M.; Murphy, D.J.; Ducharme, N.G. Negative pressure pulmonary edema as a post-anesthetic complication associated with upper airway obstruction in a horse. Vet. Surg. 1996, 25, 519–523.
- McGreevy, P.D. The fine line between pressure and pain: Ask the horse. Vet. J. 2011, 188, 250–251.
- McGreevy, P.D.; Harman, A.; McLean, A.; Hawson, L. Over-flexing the horse’s neck: A modern equestrian obsession? J. Vet. Behav. 2010, 5, 180–186.
- McLean, A.N.; McGreevy, P.D. Ethical equitation: Capping the price horses pay for human glory. J. Vet. Behav. 2010, 5, 203–209.
- McGreevy, P.D.; McLean, A.N.; Warren-Smith, A.K.; Waran, N.; Goodwin, D. Defining the terms and processes associated with equitation. In Proceedings of the 1st International Equitation Science Symposium 2005, Melbourne, Australia, 26–27 July 2005; Sydney University Press: Sydney, NSW, Australia, 2005; pp. 10–43.
- Fraser, D. Understanding Animal Welfare: The Science in its Cultural Context; Wiley-Blackwell: Oxford, UK, 2008.
- Mellor, D.J. Welfare-aligned sentience: Enhanced capacities to experience, inte ract, anticipate, choose and survive. Animals 2019, 9, 440.
- Mellor, D.J. Updating animal welfare thinking: Moving beyond the ‘five freedoms’ towards ‘a life worth living’. Animals 2016, 6, 21.




