
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yinyong Sun | + 2457 word(s) | 2457 | 2021-12-29 09:13:02 | | | |
| 2 | Conner Chen | Meta information modification | 2457 | 2022-01-19 02:59:27 | | |
Video Upload Options
Catalytic oxidative desulfurization (ODS) of fuel oils is considered one of the most promising non-hydrodesulfurization technologies due to the advantages of mild reaction conditions, low cost and easy removal of aromatic sulfur compounds. Based on this reason, the preparation of highly efficient ODS catalysts has been a hot research topic in this field. Metal-organic frameworks (MOFs) have received much attention due to advantages such as abundant metal centers, high surface areas and varied pore structures, which are composed of secondary building units (SBUs) connected by organic linkers to form crystalline porous materials. Such materials possess both the rigidity of inorganic materials and the flexibility of organic materials. Moreover, rich metal centers in the structure of MOFs could be catalytic active sites for some chemical reactions.
1. Ti-MOFs as ODS Catalysts
| Catalyst | Substrate (S Content) |
Oxidant | O/S Ratio | Temp. (°C) |
Time (min) |
Sulfur Removal (%) | Activity (mmol·g−1·h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| MIL-125(Ti) |
DBT (200 ppm) 4-MDBT (200 ppm) 4,6-DMDBT (200 ppm) |
CHP |
15 |
80 |
180 |
36 15 12 |
0.30 0.12 0.10 |
[4] |
| meso-MIL-125(Ti) | DBT (500 ppm) | TBHP | 10 | 80 | - | - | (22.9) a | [5] |
| MIL-125(Ti)-L | DBT (240 ppm) | H2O2 TBHP |
8 8 |
60 60 |
30 30 |
95.3 44.9 |
1.0 0.5 |
[6] |
| COK-47S(Ti) | DBT (3601 ppm) | TBHP | 2.5 | 60 | 120 | 99 | (41.1) a | [7] |
| Ti-BDC-180 | DBT (1000 ppm) | H2O2 | 6 | 60 | 20 | 99.8 | 18.7 | [8] |
| Ti-BDC-A | DBT (500 ppm) | CHP | 6 | 25 | 10 | 100 | 21.9 | [9] |
2. Zr-MOFs as ODS Catalysts
| Catalyst | Substrate (S Content) |
Oxidant | O/S Ratio | Temp. (°C) |
Time (min) | Sulfur Removal (%) | Activity (mmol·g−1·h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| UiO-66(Zr) UiO-66(Zr)mod UiO-66(Zr)HCl,mod UiO-66(Zr)HCl |
DBT, 4-MDBT and 4,6-DMDBT (500 ppm for each) |
H2O2 | 12 | 50 | 30 | 99.6 71.2 59.9 36.9 |
- | [11] |
| UiO-66(Zr)-1h | DBT (1000 ppm) | H2O2 | ≈11 | 60 | 60 | 97 | 8.5 | [12] |
| UiO-66(Zr)-solvent UiO-66(Zr)-free |
DBT (1000 ppm) 4,6-DMDBT (500 ppm) DBT (1000 ppm) 4,6-DMDBT (500 ppm) |
H2O2 H2O2 |
6 6 |
60 60 |
120 120 |
80.5 52.5 99.6 98.1 |
2.5 0.82 3.1 1.5 |
[15] |
| UiO-66(Zr) | DBT (500 ppm) | H2O2 | 12 | 60 | 150 | 100 | - | [17] |
| UiO-66(Zr)-NO2-green UiO-66(Zr)-NH2-green UiO-66(Zr) –green |
DBT (1000 ppm) 4,6-DMDBT (500 ppm) DBT (1000 ppm) 4,6-DMDBT (500 ppm) DBT (1000 ppm) 4,6-DMDBT (500 ppm) |
H2O2 H2O2 H2O2 |
6 6 6 |
60 60 60 |
30 30 30 |
99.6 99.6 62.6 45.6 90.3 94.2 |
- | [22] |
| UiO-66(Zr)-S1 UiO-66(Zr)-MW2 |
1-BT, DBT, 4-MDBT and 4,6-DMDBT (500 ppm for each) |
H2O2 |
13 |
50 |
180 |
99.5 96 |
- | [20] |
| UiO-66(Zr)-ZrCl4 UiO-66(Zr) |
BT, DBT, 4-MDBT and 4,6-DMDBT (500 ppm for each) |
H2O2 | 13 | 50 | 60 | 97 62 |
- | [21] |
| HP-UiO-66(Zr) | DBT (1000 ppm) 4,6-DMDBT (1000 ppm) |
H2O2 | 4 | 30 | 30 60 |
94.3 99.3 |
19.6 10.3 |
[24] |
| Ti-UiO-66-D UiO-66-D Ti-UiO-66-H UiO-66-H |
DBT (1000 ppm) DBT (1000 ppm) |
H2O2 H2O2 |
6 6 |
60 60 |
120 120 |
91.7 50.7 66.3 5.6 |
2.9 1.6 2.1 0.17 |
[25] |
| UiO-66(0.13Hf-Zr) |
DBT (1000 ppm) BT (1000 ppm) 4,6-DMDBT (1000 ppm) |
H2O2 |
4 |
30 |
15 |
99.8 70.8 100 |
17.5 - - |
[27] |
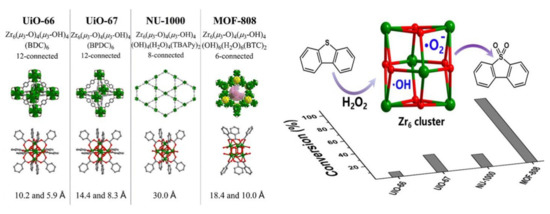
| UiO-66(Zr) UiO-67(Zr) NU-1000(Zr) MOF-808(Zr) |
DBT (1000 ppm) DBT (1000 ppm) DBT (1000 ppm) DBT (1000 ppm) |
H2O2 H2O2 H2O2 H2O2 |
5 5 5 5 |
50 50 50 50 |
5 5 5 5 |
8.8 20.8 11.1 100 |
- | [30] |
| NU-1000(Zr) | DBT (1000 ppm) BT (500 ppm) 3-MDBT (500 ppm) 4,6-DMDBT (500 ppm) |
H2O2 |
6 |
60 |
180 |
100 ≈62 ≈81 67.6 |
2.6 - - - |
[32] |
| UMCM-309(Zr) MOF-808(Zr)-M |
DBT (4319 ppm) BT (4378 ppm) 4,6-DMDBT (4303 ppm) BT, DBT and 4,6-DMDBT (500 ppm for each) |
TBHP TBHP |
2.5 2.5 |
60 60 |
480 60 |
96 49 30 68 |
2.5 1.3 0.79 9.2 |
[28] |
| MOF-808(Zr)-H | DBT (1000 ppm) BT (1000 ppm) 4,6-DMDBT (500 ppm) |
CHP | 3 | 50 | 20 | 93 ≈34 ≈17 |
17.4 - - |
[34] |
3. Other Metal-Centered MOFs as ODS Catalysts
| Catalyst | Substrate (S Content) |
Oxidant | O/S Ratio | Temp. (°C) |
Time (min) | Sulfur Removal (%) |
Activity (mmol·g−1·h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| MIL-47(V) | DBT (15691 ppm) T (7165 ppm) BT (11428 ppm) |
TBHP |
2.15 |
80 |
- | - | (29) a (1.3) a (9.9) a |
[35] |
| TMU-10(Co) TMU-12(Co) |
DBT (500 ppm) DBT (500 ppm) |
TBHP TBHP |
3 3 |
60 60 |
360 360 |
40.5 75.2 |
0.17 0.32 |
[40] |
| NH2–TMU(Co)-53 | DBT (500 ppm) | H2O2 | 3 | 60 | 120 | 79.4 | (10.6) a | [41] |
| MIL-101(Cr) | DBT (1534 ppm) | O2 | - | 120 | 1260 | 99.6 | - | [42] |
| MIL-101(Cr)-NO2 | DBT (200 ppm) | O2 | - | 140 | ≈270 | ≈100 | - | [43] |
| MFM-300(V) | DBT (200 ppm) BT (200 ppm) 4,6-DMDBT (200 ppm) |
Air | - | 120 | 300 | 99.6 18.0 98.1 |
0.62 - - |
[38] |
References
- Jin, C.; Li, G.; Wang, X.; Zhao, L.; Liu, L.; Liu, H.; Liu, Y.; Zhang, W.; Han, X.; Bao, X. Synthesis, Characterization and Catalytic Performance of Ti-Containing Mesoporous Molecular Sieves Assembled from Titanosilicate Precursors. Chem. Mater. 2007, 19, 1664–1670.
- Kim, T.-W.; Kim, M.-J.; Kleitz, F.; Nair, M.M.; Guillet-Nicolas, R.; Jeong, K.-E.; Chae, H.-J.; Kim, C.-U.; Jeong, S.-Y. Tailor-Made Mesoporous Ti-SBA-15 Catalysts for Oxidative Desulfurization of Refractory Aromatic Sulfur Compounds in Transport Fuel. ChemCatChem 2012, 4, 687–697.
- Dan-Hardi, M.; Serre, C.; Frot, T.; Rozes, L.; Maurin, G.; Sanchez, C.; Ferey, G. A new photoactive crystalline highly porous titanium(IV) dicarboxylate. J. Am. Chem. Soc. 2009, 131, 10857–10859.
- Kim, S.-N.; Kim, J.; Kim, H.-Y.; Cho, H.-Y.; Ahn, W.-S. Adsorption/catalytic properties of MIL-125 and NH2-MIL-125. Catal. Today 2013, 204, 85–93.
- McNamara, N.D.; Hicks, J.C. Chelating agent-free, vapor-assisted crystallization method to synthesize hierarchical microporous/mesoporous MIL-125 (Ti). ACS Appl. Mater. Interfaces 2015, 7, 5338–5346.
- Zhang, Y.; Li, G.; Kong, L.; Lu, H. Deep oxidative desulfurization catalyzed by Ti-based metal-organic frameworks. Fuel 2018, 219, 103–110.
- Smolders, S.; Willhammar, T.; Krajnc, A.; Sentosun, K.; Wharmby, M.T.; Lomachenko, K.A.; Bals, S.; Mali, G.; Roeffaers, M.B.J.; De Vos, D.E.; et al. A Titanium(IV)-Based Metal-Organic Framework Featuring Defect-Rich Ti-O Sheets as an Oxidative Desulfurization Catalyst. Angew. Chem. Int. Ed. Engl. 2019, 58, 9160–9165.
- Ye, G.; Sun, Y.; Zhang, D.; Zhou, W.; Lancelot, C.; Rives, A.; Lamonier, C.; Xu, W. Hierarchical porous titanium terephthalate based material with highly active sites for deep oxidative desulfurization. Microporous Mesoporous Mater. 2018, 270, 241–247.
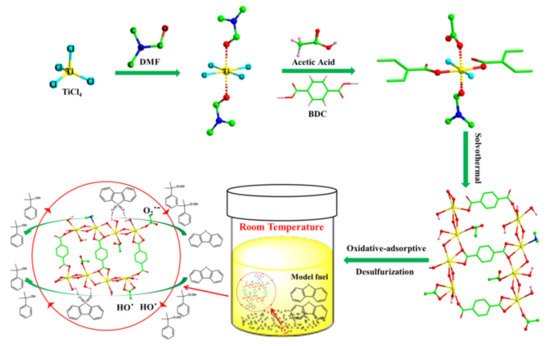
- Ye, G.; Gu, Y.; Zhou, W.; Xu, W.; Sun, Y. Synthesis of Defect-Rich Titanium Terephthalate with the Assistance of Acetic Acid for Room-Temperature Oxidative Desulfurization of Fuel Oil. ACS Catal. 2020, 10, 2384–2394.
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851.
- Granadeiro, C.M.; Ribeiro, S.O.; Karmaoui, M.; Valenca, R.; Ribeiro, J.C.; de Castro, B.; Cunha-Silva, L.; Balula, S.S. Production of ultra-deep sulfur-free diesels using a sustainable catalytic system based on UiO-66(Zr). Chem. Commun. 2015, 51, 13818–13821.
- Xiao, W.; Dong, Q.; Wang, Y.; Li, Y.; Deng, S.; Zhang, N. Time modulation of defects in UiO-66 and application in oxidative desulfurization. CrystEngComm 2018, 20, 5658–5662.
- Zhuang, S.; Cheng, R.; Wang, J. Adsorption of diclofenac from aqueous solution using UiO-66-type metal-organic frameworks. Chem. Eng. J. 2019, 359, 354–362.
- Dhakshinamoorthy, A.; Santiago-Portillo, A.; Asiri, A.M.; Garcia, H. Engineering UiO-66 Metal Organic Framework for Heterogeneous Catalysis. ChemCatChem 2019, 11, 899–923.
- Ye, G.; Zhang, D.; Li, X.; Leng, K.; Zhang, W.; Ma, J.; Sun, Y.; Xu, W.; Ma, S. Boosting Catalytic Performance of Metal-Organic Framework by Increasing the Defects via a Facile and Green Approach. ACS Appl. Mater. Interfaces 2017, 9, 34937–34943.
- Vermoortele, F.; Ameloot, R.; Vimont, A.; Serre, C.; De Vos, D. An amino-modified Zr-terephthalate metal-organic framework as an acid-base catalyst for cross-aldol condensation. Chem. Commun. 2011, 47, 1521–1523.
- Zhang, X.; Huang, P.; Liu, A.; Zhu, M. A metal–organic framework for oxidative desulfurization: UIO-66(Zr) as a catalyst. Fuel 2017, 209, 417–423.
- Vermoortele, F.; Vandichel, M.; Van de Voorde, B.; Ameloot, R.; Waroquier, M.; Van Speybroeck, V.; De Vos, D.E. Electronic effects of linker substitution on Lewis acid catalysis with metal-organic frameworks. Angew. Chem. Int. Ed. Engl. 2012, 51, 4887–4890.
- Peng, Y.; Huang, H.; Zhang, Y.; Kang, C.; Chen, S.; Song, L.; Liu, D.; Zhong, C. A versatile MOF-based trap for heavy metal ion capture and dispersion. Nat. Commun. 2018, 9, 187.
- Viana, A.M.; Ribeiro, S.O.; Castro, B.; Balula, S.S.; Cunha-Silva, L. Influence of UiO-66(Zr) Preparation Strategies in Its Catalytic Efficiency for Desulfurization Process. Materials 2019, 12, 3009.
- Viana, A.M.; Julião, D.; Mirante, F.; Faria, R.G.; de Castro, B.; Balula, S.S.; Cunha-Silva, L. Straightforward activation of metal-organic framework UiO-66 for oxidative desulfurization processes. Catal. Today 2021, 362, 28–34.
- Ye, G.; Qi, H.; Zhou, W.; Xu, W.; Sun, Y. Green and scalable synthesis of nitro- and amino-functionalized UiO-66(Zr) and the effect of functional groups on the oxidative desulfurization performance. Inorg. Chem. Front. 2019, 6, 1267–1274.
- Yang, D.; Gates, B.C. Catalysis by Metal Organic Frameworks: Perspective and Suggestions for Future Research. ACS Catal. 2019, 9, 1779–1798.
- Hao, L.; Stoian, S.A.; Weddle, L.R.; Zhang, Q. Zr-Based MOFs for oxidative desulfurization: What matters? Green Chem. 2020, 22, 6351–6356.
- Ye, G.; Qi, H.; Li, X.; Leng, K.; Sun, Y.; Xu, W. Enhancement of Oxidative Desulfurization Performance over UiO-66(Zr) by Titanium Ion Exchange. Chemphyschem 2017, 18, 1903–1908.
- He, Y.; Wu, Y.; Chen, T.; Weng, W.; Wan, H. Low-temperature catalytic performance for oxidative dehydrogenation of propane on nanosized Ti(Zr)–Ni–O prepared by modified sol–gel method. Catal. Commun. 2006, 7, 268–271.
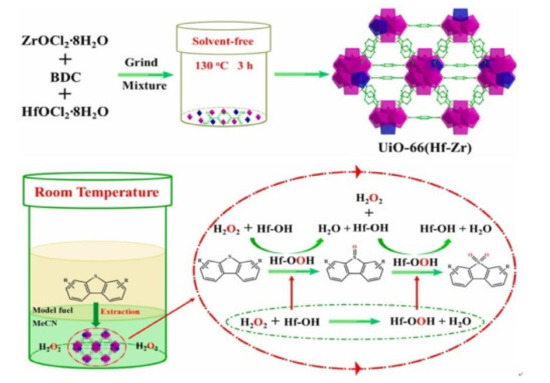
- Ye, G.; Wang, H.; Zeng, X.; Wang, L.; Wang, J. Defect-rich bimetallic UiO-66(Hf-Zr): Solvent-free rapid synthesis and robust ambient-temperature oxidative desulfurization performance. Appl. Catal. B Environ. 2021, 299, 120659.
- Fu, G.; Bueken, B.; De Vos, D. Zr-Metal-Organic Framework Catalysts for Oxidative Desulfurization and Their Improvement by Postsynthetic Ligand Exchange. Small Methods 2018, 2, 1800203.
- Wang, J.C.; Ding, F.W.; Ma, J.P.; Liu, Q.K.; Cheng, J.Y.; Dong, Y.B. Co(II)-MOF: A Highly Efficient Organic Oxidation Catalyst with Open Metal Sites. Inorg. Chem. 2015, 54, 10865–10872.
- Zheng, H.Q.; Zeng, Y.N.; Chen, J.; Lin, R.G.; Zhuang, W.E.; Cao, R.; Lin, Z.J. Zr-Based Metal-Organic Frameworks with Intrinsic Peroxidase-Like Activity for Ultradeep Oxidative Desulfurization: Mechanism of H2O2 Decomposition. Inorg. Chem. 2019, 58, 6983–6992.
- Ji, P.; Drake, T.; Murakami, A.; Oliveres, P.; Skone, J.H.; Lin, W. Tuning Lewis Acidity of Metal-Organic Frameworks via Perfluorination of Bridging Ligands: Spectroscopic, Theoretical, and Catalytic Studies. J. Am. Chem. Soc. 2018, 140, 10553–10561.
- Hao, L.; Hurlock, M.J.; Li, X.; Ding, G.; Kriegsman, K.W.; Guo, X.; Zhang, Q. Efficient oxidative desulfurization using a mesoporous Zr-based MOF. Catal. Today 2020, 350, 64–70.
- Mondloch, J.E.; Bury, W.; Fairen-Jimenez, D.; Kwon, S.; DeMarco, E.J.; Weston, M.H.; Sarjeant, A.A.; Nguyen, S.T.; Stair, P.C.; Snurr, R.Q.; et al. Vapor-phase metalation by atomic layer deposition in a metal-organic framework. J. Am. Chem. Soc. 2013, 135, 10294–10297.
- Gu, Y.; Ye, G.; Xu, W.; Zhou, W.; Sun, Y. Creation of Active Sites in MOF-808(Zr) by a Facile Route for Oxidative Desulfurization of Model Diesel Oil. ChemistrySelect 2020, 5, 244–251.
- McNamara, N.D.; Neumann, G.T.; Masko, E.T.; Urban, J.A.; Hicks, J.C. Catalytic performance and stability of (V) MIL-47 and (Ti) MIL-125 in the oxidative desulfurization of heterocyclic aromatic sulfur compounds. J. Catal. 2013, 305, 217–226.
- Wang, D.; Qian, E.W.; Amano, H.; Okata, K.; Ishihara, A.; Kabe, T. Oxidative desulfurization of fuel oil. Appl. Catal. A Gen. 2003, 253, 91–99.
- Otsuki, S.; Nonaka, T.; Takashima, N.; Qian, W.; Ishihara, A.; Imai, T.; Kabe, T. Oxidative Desulfurization of Light Gas Oil and Vacuum Gas Oil by Oxidation and Solvent Extraction. Energy Fuels 2000, 14, 1232–1239.
- Li, X.; Gu, Y.; Chu, H.; Ye, G.; Zhou, W.; Xu, W.; Sun, Y. MFM-300(V) as an active heterogeneous catalyst for deep desulfurization of fuel oil by aerobic oxidation. Appl. Catal. A Gen. 2019, 584, 117152.
- Lu, Z.; Godfrey, H.G.; da Silva, I.; Cheng, Y.; Savage, M.; Tuna, F.; McInnes, E.J.; Teat, S.J.; Gagnon, K.J.; Frogley, M.D.; et al. Modulating supramolecular binding of carbon dioxide in a redox-active porous metal-organic framework. Nat. Commun. 2017, 8, 14212.
- Masoomi, M.Y.; Bagheri, M.; Morsali, A. Application of Two Cobalt-Based Metal-Organic Frameworks as Oxidative Desulfurization Catalysts. Inorg. Chem. 2015, 54, 11269–11275.
- Abazari, R.; Sanati, S.; Morsali, A.; Slawin, A.; Carpenter-Warren, C.L. Dual-Purpose 3D Pillared Metal-Organic Framework with Excellent Properties for Catalysis of Oxidative Desulfurization and Energy Storage in Asymmetric Supercapacitor. ACS Appl. Mater. Interfaces 2019, 11, 14759–14773.
- Gómez-Paricio, A.; Santiago-Portillo, A.; Navalón, S.; Concepción, P.; Alvaro, M.; Garcia, H. MIL-101 promotes the efficient aerobic oxidative desulfurization of dibenzothiophenes. Green Chem. 2016, 18, 508–515.
- Vallés-García, C.; Santiago-Portillo, A.; Álvaro, M.; Navalón, S.; García, H. MIL-101(Cr)-NO2 as efficient catalyst for the aerobic oxidation of thiophenols and the oxidative desulfurization of dibenzothiophenes. Appl. Catal. A Gen. 2020, 590, 117340.




