Catalytic oxidative desulfurization (ODS) of fuel oils is considered one of the most promising non-hydrodesulfurization technologies due to the advantages of mild reaction conditions, low cost and easy removal of aromatic sulfur compounds. Based on this reason, the preparation of highly efficient ODS catalysts has been a hot research topic in this field. Metal-organic frameworks (MOFs) have received much attention due to advantages such as abundant metal centers, high surface areas and varied pore structures, which are composed of secondary building units (SBUs) connected by organic linkers to form crystalline porous materials. Such materials possess both the rigidity of inorganic materials and the flexibility of organic materials. Moreover, rich metal centers in the structure of MOFs could be catalytic active sites for some chemical reactions.
1. Ti-MOFs as ODS Catalysts
In the earlier studies, the Ti species in titanium-containing zeolites and mesoporous silica have been demonstrated to be active in the ODS reactions of sulfur-containing compounds [
20,
21]. However, the content of the introduced Ti species into the framework is low (<5 wt.%) due to the limitation of the structure. Compared with these Ti-containing porous materials, Ti-MOFs are promising candidates for ODS reactions due to the presence of rich Ti sites in their structure. As a member of Ti-MOFs, MIL-125(Ti) is attractive. In its structure, the basic unit of MIL-125(Ti) is Ti
8O
8(OH)
4-(O
2C-C
6H
5-CO
2)
6 and a cyclic octamer is composed of titanium octahedral units shared by corners or edges. These cyclic octamers are connected to the other 12 cyclic octamers through BDC linkers to form a porous three-dimensional periodic array with two types of cages. One type of cage is an octahedron (12.5 Å) and the other is a tetrahedron (6 Å) [
22].
In 2013, Se-Na Kim and coworkers first reported that MIL-125(Ti) was active in the oxidative desulfurization of DBT [
23]. In this work, the catalytic ODS performance of MIL-125(Ti) was evaluated and compared with traditional microporous and mesoporous titanium silicates. The results showed that the order of DBT conversion under the same reaction conditions was Meso-TS-1 > MIL-125(Ti) > Micro-TS-1 (
Table 1), which is in accordance with the window size in porous materials. This result suggests that pore size also plays an important role in the ODS reactions of sulfur compounds besides Ti species. To overcome the limitation of micropore size, meso-MIL-125(Ti) with mesopores was synthesized in the presence of surfactant by vapor-assisted crystallization method [
24]. The catalytic results further demonstrate that the introduction of mesopores could improve the catalytic ODS performance of MIL-125(Ti). Additionally, Li et al. investigated the catalytic performance of MIL-125(Ti) and NH
2-MIL-125(Ti) with different crystal sizes; MIL-125(Ti)-L with large crystal size showed the best catalytic performance when H
2O
2 was used as oxidant [
25]. It is thought that MIL-125(Ti) with large crystal size hould possess more coordination-unsaturated Ti(IV) sites, which is more favorable for catalytic oxidative desulfurization. Although some progress on MIL-125(Ti) has been made, its structural stability is still an issue during the ODS reactions.
Table 1. Comparison of catalytic performance over various Ti-MOFs in the ODS reactions of model fuel oil.
| Catalyst |
Substrate
(S Content) |
Oxidant |
O/S Ratio |
Temp.
(°C) |
Time
(min) |
Sulfur Removal (%) |
Activity (mmol·g−1·h−1) |
Ref. |
MIL-125(Ti)
|
DBT (200 ppm)
4-MDBT (200 ppm)
4,6-DMDBT (200 ppm) |
CHP
|
15
|
80
|
180
|
36
15
12 |
0.30
0.12
0.10 |
[23] |
| meso-MIL-125(Ti) |
DBT (500 ppm) |
TBHP |
10 |
80 |
- |
- |
(22.9) a |
[24] |
| MIL-125(Ti)-L |
DBT (240 ppm) |
H2O2
TBHP |
8
8 |
60
60 |
30
30 |
95.3
44.9 |
1.0
0.5 |
[25] |
| COK-47S(Ti) |
DBT (3601 ppm) |
TBHP |
2.5 |
60 |
120 |
99 |
(41.1) a |
[26] |
| Ti-BDC-180 |
DBT (1000 ppm) |
H2O2 |
6 |
60 |
20 |
99.8 |
18.7 |
[27] |
| Ti-BDC-A |
DBT (500 ppm) |
CHP |
6 |
25 |
10 |
100 |
21.9 |
[28] |
Recently, Simon Smolders et al. synthesized a new type of Ti-MOF (COK-47) [
26]. This material used the composite layer of TiO
6 octahedron as two-dimensional secondary structural units to form a three-dimensional framework through a bpdc
2− linkers connection. The catalytic results showed that 99% DBT over COK-47
S was oxidized in 120 min at 60 °C by TBHP as an oxidant. Such catalytic performance could be attributed to the presence of many methoxy groups (Me-O-Ti) and open-metal Ti sites. Moreover, the catalyst remained structurally stable after three recycles.
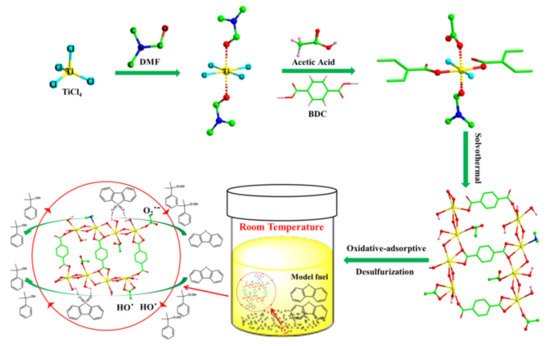
More recently, Sun and coworkers reported that new porous titanium terephthalates with hierarchical porosity (Ti-BDC) and amorphous nature have been successfully synthesized for deep oxidative desulfurization of fuel oil [
27]. The catalytic results indicate that Ti-BDC exhibited superior catalytic activity in the ODS reactions of BT, DBT and 4,6-DMDBT than MIL-125(Ti) and meso-MIL-125(Ti), which might be attributed to the introduction of partial mesopores and abundant coordination-unsaturated Ti sites. Especially in the above synthetic system, a novel porous titanium terephthalate (Ti-BDC-A) with more defective Ti sites and higher surface area was produced with the assistance of acetic acid (
Figure 1). When the material is used as an ODS catalyst, DBT (500 ppm) in model fuel oil can be completely oxidized in 10 min at room temperature [
28].
Figure 1. Imaginative diagram of the formation process of Ti-BDC-A and proposed reaction mechanism of the catalytic ODS of DBT over Ti-BDC-A (reproduced from Ref. [
28], copyright 2020 American Chemical Society).
2. Zr-MOFs as ODS Catalysts
It is generally well known that Zr-MOFs possess high structural stability compared with other metal-based MOFs. One example is UiO-66 (Zr), whose structure composed of Zr
6O
4(μ
3-OH)
4 clusters in coordination with 12 terephthalic acids [
29]. In 2015, Granadeiro et al. first reported that UiO-66(Zr) as a heterogeneous catalyst was used in the ODS of diesel. In this work, UiO-66(Zr) samples were prepared by different synthesis methods and their catalytic ODS performance were evaluated [
30]. It is noted that the catalytic performance had a close relationship with the crystallinity degree and linker defects in UiO-66 (Zr). The samples with low crystallinity displayed superior catalytic performance. Meanwhile, the good catalytic activity can be kept in the dual phase catalytic system with H
2O
2 as an oxidant and acetonitrile as an extractant for the ODS of model and real diesel. Similarly, some researchers obtained UiO-66(Zr) with lower crystallinity by shortening the synthesis time, but not surprisingly its reusability was poor [
31]. A detailed comparison of catalytic performance over various Zr-MOFs is shown in
Table 2.
In general, the defects in crystal materials are closely related to their properties. Thus, it is of great importance to prepare the defective crystal materials [
32,
33,
34,
35,
36,
37,
38,
39,
40]. As for UiO-66(Zr), some methods have been developed to fabricate defective UiO-66(Zr). For example, Sun et al. adopted solvent-free synthesis to produce defective UiO-66(Zr) [
34]. The catalytic data indicate that UiO-66(Zr) free with rich defects exhibited much better catalytic activity than UiO-66(Zr) solvent with high crystallinity prepared by conventional solvothermal route in the ODS reactions. Such catalytic performance has a good correlation with the number of Lewis acid sites in UiO-66(Zr) because the defects that resulted from the loss of organic linkers may promote the formation of Lewis acid sites [
35,
36]. Based on this study, this research group synthesized defective amino- and nitro-functionalized UiO-66(Zr) by solvent-free method. The results showed that nitro-functionalized UiO-66(Zr) exhibited excellent catalytic activity in the oxidative desulfurization of DBT and 4,6-DMDBT. The ODS activity could almost be kept after five cycles [
17]. The possible reason is that the introduction of electron-withdrawing groups reduces the electron density around Zr and enhances its electron-withdrawing ability [
37,
38]. On the other hand, most of MOFs are microporous materials, which would limit the mass transfer of reactants and hinder the accessibility of catalytic active sites in the pores [
41]. For this, Hao et al. synthesized hierarchical porous UiO-66(Zr) (HP-UiO-66(Zr) and evaluated its ODS performance. The results showed that HP-UiO-66(Zr) could complete the oxidation of DBT and 4,6-DMDBT at room temperature at a low oxidant dosage (O/S = 4) [
42].
Based on the structure of UiO-66(Zr), bimetal-centered UiO-66(Zr) materials were also prepared. Sun et al. firstly reported Ti-UiO-66(Zr) obtained by ion-exchange method for ODS reactions [
43]. It is well known that Ti ions have stronger oxidation ability than Zr ions [
44]. Consequently, Ti-UiO-66(Zr) showed better catalytic ODS performance than the parent UiO-66(Zr). In addition, Ye et al. prepared Hf-incorporated UiO-66(Zr) under solvent-free conditions [
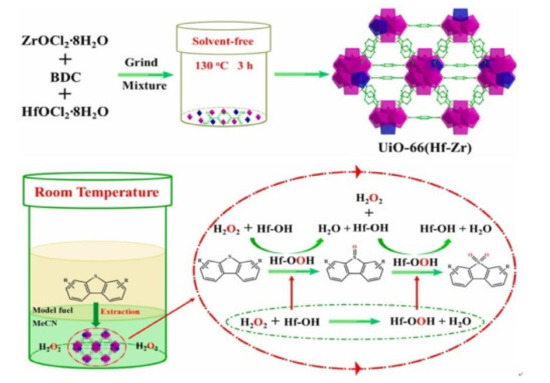
45]. In the structure of this material, 0.7 Zr atoms are replaced by Hf atoms to form Zr-Hf-oxo clusters with a considerable number of Zr/Hf-OH active sites (
Figure 2). The obtained catalyst may complete the oxidation of 4,6-DMDBT (1000 ppm) from model oil within 15 min at room temperature. Theoretical studies indicate that the exposed Hf-OH centers can easily react with H
2O
2 to form Hf-OOH intermediates and thus control the reactivity.
Figure 2. Schematic illustration about the synthetic process of UiO-66(0.13Hf-Zr) and proposed ODS reaction mechanism over UiO-66(0.13Hf-Zr) (adapted from Ref. [
45], copyright 2021 Elsevier).
Besides UiO-66(Zr), other research works about Zr-MOFs involve two-dimensional layered UMCM-309(Zr) and three-dimensional porous MOF-808(Zr) with the same secondary structure units [Zr
6O
4(OH)
4(−COO)
6]
6+. The study from Fu et al. showed that MOF-808(Zr) had better ODS activity than UMCM-309(Zr). Further, the catalytic activity over MOF-808(Zr) can be greatly improved after the removal of partial formic acid in its structure because more open metal sites are generated [
46,
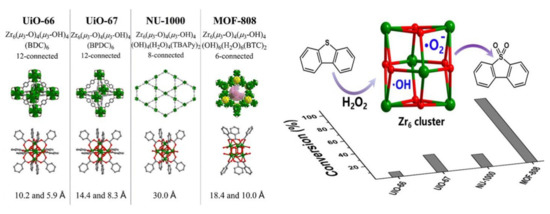
47]. Some studies suggest that the order of catalytic ODS activity over different Zr-MOFs is MOF-808(Zr) > UiO-67(Zr) > NU-1000(Zr) > UiO-66(Zr), which coincides with the number of Lewis acid sites (
Figure 3) [
48,
49]. Moreover, the studies on NU-1000(Zr) and MOF-808(Zr) revealed that mesoporous structure and Lewis acidity played an important role on the ODS activity of Zr-MOF materials [
50,
51,
52].
Table 2. Comparison of catalytic performance over various Zr-MOFs in the ODS reactions of model fuel oil.
| Catalyst |
Substrate
(S Content) |
Oxidant |
O/S Ratio |
Temp.
(°C) |
Time (min) |
Sulfur Removal (%) |
Activity (mmol·g−1·h−1) |
Ref. |
UiO-66(Zr)
UiO-66(Zr)mod
UiO-66(Zr)HCl,mod
UiO-66(Zr)HCl |
DBT, 4-MDBT
and 4,6-DMDBT
(500 ppm for each) |
H2O2 |
12 |
50 |
30 |
99.6
71.2
59.9
36.9 |
- |
[30] |
| UiO-66(Zr)-1h |
DBT (1000 ppm) |
H2O2 |
≈11 |
60 |
60 |
97 |
8.5 |
[31] |
UiO-66(Zr)-solvent
UiO-66(Zr)-free |
DBT (1000 ppm)
4,6-DMDBT (500 ppm)
DBT (1000 ppm)
4,6-DMDBT (500 ppm) |
H2O2
H2O2 |
6
6 |
60
60 |
120
120 |
80.5
52.5
99.6
98.1 |
2.5
0.82
3.1
1.5 |
[34] |
| UiO-66(Zr) |
DBT (500 ppm) |
H2O2 |
12 |
60 |
150 |
100 |
- |
[36] |
UiO-66(Zr)-NO2-green
UiO-66(Zr)-NH2-green
UiO-66(Zr) –green |
DBT (1000 ppm)
4,6-DMDBT (500 ppm)
DBT (1000 ppm)
4,6-DMDBT (500 ppm)
DBT (1000 ppm)
4,6-DMDBT (500 ppm) |
H2O2
H2O2
H2O2 |
6
6
6 |
60
60
60 |
30
30
30 |
99.6
99.6
62.6
45.6
90.3
94.2 |
- |
[17] |
UiO-66(Zr)-S1
UiO-66(Zr)-MW2 |
1-BT, DBT, 4-MDBT
and 4,6-DMDBT
(500 ppm for each) |
H2O2
|
13
|
50
|
180
|
99.5
96 |
- |
[39] |
UiO-66(Zr)-ZrCl4
UiO-66(Zr) |
BT, DBT, 4-MDBT
and 4,6-DMDBT
(500 ppm for each) |
H2O2 |
13 |
50 |
60 |
97
62 |
- |
[40] |
| HP-UiO-66(Zr) |
DBT (1000 ppm)
4,6-DMDBT (1000 ppm) |
H2O2 |
4 |
30 |
30
60 |
94.3
99.3 |
19.6
10.3 |
[42] |
Ti-UiO-66-D
UiO-66-D
Ti-UiO-66-H
UiO-66-H |
DBT (1000 ppm)
DBT (1000 ppm) |
H2O2
H2O2 |
6
6 |
60
60 |
120
120 |
91.7
50.7
66.3
5.6 |
2.9
1.6
2.1
0.17 |
[43] |
UiO-66(0.13Hf-Zr)
|
DBT (1000 ppm)
BT (1000 ppm)
4,6-DMDBT (1000 ppm) |
H2O2
|
4
|
30
|
15
|
99.8
70.8
100 |
17.5
-
- |
[45] |
UiO-66(Zr)
UiO-67(Zr)
NU-1000(Zr)
MOF-808(Zr) |
DBT (1000 ppm)
DBT (1000 ppm)
DBT (1000 ppm)
DBT (1000 ppm) |
H2O2
H2O2
H2O2
H2O2 |
5
5
5
5 |
50
50
50
50 |
5
5
5
5 |
8.8
20.8
11.1
100 |
- |
[48] |
| NU-1000(Zr) |
DBT (1000 ppm)
BT (500 ppm)
3-MDBT (500 ppm)
4,6-DMDBT (500 ppm) |
H2O2
|
6
|
60
|
180
|
100
≈62
≈81
67.6 |
2.6
-
-
- |
[50] |
UMCM-309(Zr)
MOF-808(Zr)-M
|
DBT (4319 ppm)
BT (4378 ppm)
4,6-DMDBT (4303 ppm)
BT, DBT and 4,6-DMDBT
(500 ppm for each) |
TBHP
TBHP
|
2.5
2.5
|
60
60
|
480
60
|
96
49
30
68
|
2.5
1.3
0.79
9.2
|
[46] |
| MOF-808(Zr)-H |
DBT (1000 ppm)
BT (1000 ppm)
4,6-DMDBT (500 ppm) |
CHP |
3 |
50 |
20 |
93
≈34
≈17 |
17.4
-
- |
[52] |
Figure 3. The structure of various Zr-MOFs and their catalytic oxidation performance (adapted from Ref. [
48], copyright 2019 American Chemical Society).
3. Other Metal-Centered MOFs as ODS Catalysts
The other metal-centered MOFs mainly include the studies of V-MOFs, Co-MOFs and MIL-101(Fe/Cr) for ODS reactions. A detailed comparison of catalytic performance over other metal-centered MOFs in the ODS reactions of model fuel oil is shown in Table 3.
MIL-47(V) is the first example of non-Zr- and Ti-based MOFs for ODS reactions. In this work, the catalytic performance of MIL-47(V) was compared with that of MIL-125(Ti) by the oxidation reactions of T, BT and DBT [
53]. The results show that MIL-47(V) exhibited better catalytic ODS performance than MIL-125(Ti) in the oxidation of DBT. Meanwhile, MIL-47(V) is more suitable for the catalytic oxidation of DBT at relatively low reaction temperatures, which should be related to the pore size of the material. Under the same reaction conditions, the removal efficiency of BT over MIL-47(V) is not as good as that of DBT. A reasonable explanation is that the electron density of sulfur atoms in DBT is higher, while the sulfur species with higher electron density have higher catalytic activity [
54,
55]. Notably, MIL-47(V) exhibited unusual catalytic activity in the oxidation of thiophene, suggesting that metal centers in MOFs have a great influence on the ODS reactions. Although the catalytic ODS performance of MIL-47(V) is better than that of MIL-125(Ti), its structural stability is poor.
Another interesting study about V-MOFs is the study on MFM-300(V). Li et al. found that MFM-300(V) can carry out the ODS reaction by ambient air as the oxidant, and the removal of DBT and 4,6-DMDBT reached 99.6% and 98.1%, respectively [
56]. The bridging oxygen atoms in the structure exist on the corner of pores and make MFM-300(V) surface of pores locally electron-rich, which is conducive to capturing protons from hydrocarbons and activating oxygen in the air [
57].
Masoomi et al. reported that Co-MOF materials (TMU-10 and TMU-12) were successfully synthesized and their catalytic performance in ODS reactions was evaluated [
58]. The removal of DBT (500 ppm) over TMU-12(Co) was 75.2% at 60 °C. Additionally, Abazari et al. studied the catalytic ODS performance over another type of Co-MOF (NH
2-TMU-53). The results showed that the sulfur content in a model oil for DBT removal can be reduced from 500 ppm to 103 ppm at 60 °C after 2 h [
59]. Meanwhile, it is noted that the introduction of amino functional groups may significantly improve the adsorption ability for DBT, and the oxidation rate of DBT increased with adsorption amount.
MIL-101 is an important class of MOFs, which generally possess high surface area and large pore openings. Adrián Gómez-Paricio et al. first investigated the catalytic performance of MIL-101(Fe) and MIL-101(Cr) in ODS of DBT with oxygen as the oxidant [
60]. During the reaction, an obvious induction period of 6 h was observed. Further studies disclosed that the induction period of the reaction was related to solvent diffusion and the formation of the first reactive oxygen species. The catalytic results showed that MIL-101(Cr) had better catalytic activity and cycle stability than MIL-101(Fe). Subsequently, the catalytic performance of functionalized MIL-101(Cr) was also studied. It was found that nitro-functionalized MIL-101(Cr) had better ODS activity for DBT in fuel oil [
61], which is in agreement with the case of functionalized UiO-66(Zr).
Table 3. Comparison of catalytic performance over other metal-centered MOFs in the ODS reactions of model fuel oil.
| Catalyst |
Substrate
(S Content) |
Oxidant |
O/S Ratio |
Temp.
(°C) |
Time (min) |
Sulfur
Removal
(%) |
Activity (mmol·g−1·h−1) |
Ref. |
| MIL-47(V) |
DBT (15691 ppm)
T (7165 ppm)
BT (11428 ppm) |
TBHP
|
2.15
|
80
|
- |
- |
(29) a
(1.3) a
(9.9) a |
[53] |
TMU-10(Co)
TMU-12(Co) |
DBT (500 ppm)
DBT (500 ppm) |
TBHP
TBHP |
3
3 |
60
60 |
360
360 |
40.5
75.2 |
0.17
0.32 |
[58] |
| NH2–TMU(Co)-53 |
DBT (500 ppm) |
H2O2 |
3 |
60 |
120 |
79.4 |
(10.6) a |
[59] |
| MIL-101(Cr) |
DBT (1534 ppm) |
O2 |
- |
120 |
1260 |
99.6 |
- |
[60] |
| MIL-101(Cr)-NO2 |
DBT (200 ppm) |
O2 |
- |
140 |
≈270 |
≈100 |
- |
[61] |
| MFM-300(V) |
DBT (200 ppm)
BT (200 ppm)
4,6-DMDBT (200 ppm) |
Air |
- |
120 |
300 |
99.6
18.0
98.1 |
0.62
-
- |
[56] |
This entry is adapted from the peer-reviewed paper 10.3390/catal11121557