Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daniel Méndez | + 3499 word(s) | 3499 | 2022-01-06 03:09:45 | | | |
| 2 | Vivi Li | Meta information modification | 3499 | 2022-01-18 07:25:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Méndez, D. Plectranthus neochilus Schltr. Encyclopedia. Available online: https://encyclopedia.pub/entry/18382 (accessed on 07 February 2026).
Méndez D. Plectranthus neochilus Schltr. Encyclopedia. Available at: https://encyclopedia.pub/entry/18382. Accessed February 07, 2026.
Méndez, Daniel. "Plectranthus neochilus Schltr" Encyclopedia, https://encyclopedia.pub/entry/18382 (accessed February 07, 2026).
Méndez, D. (2022, January 18). Plectranthus neochilus Schltr. In Encyclopedia. https://encyclopedia.pub/entry/18382
Méndez, Daniel. "Plectranthus neochilus Schltr." Encyclopedia. Web. 18 January, 2022.
Copy Citation
Plectranthus neochilus Schltr. (Lamiaceae) is a plant recently introduced in Cuba. Worldwide, it is an ethnomedicinal alternative for its use against microbial infections, but the Cuban population use the extracts to treat sleep disorders. To address this apparent incongruity, four collections (from different seasonal conditions in the year) of Cuban P. neochilus cultivars were analyzed in terms of their pharmacognostic characteristics.
Plectranthus neochilus
diterpenes
antimicrobial
sedative
essential oil
1. Introduction
The World Health Organization (WHO) highlights that about 80% of the population from developing countries make use of medicinal plant remedies as a cost-effective way to solve health problems. Low-income countries have limited access to expensive commercial drugs on the international market, which forces people to resolve health issues with natural herbal plants/remedies [1]. In this context, natural products or natural product derivatives continue to lead the process of obtaining new entities in drug discovery. In fact, of the 1881 new drugs approved in the period between 1981 and 2019, 787 (41.8%) are directly related to a natural origin. In addition, 489 analogs were synthesized that mimic natural compounds, which together represent two-thirds of the novel drugs [2]. The importance of natural products in health management also becomes apparent from the average growth rate of the global trade of medicinal herbal derivatives, reaching an estimated market of USD 83 billion in 2014 [3]. Therefore, natural medicines should be the focus of underdeveloped countries not only as a way to solve their own health problems but also to get access to new income sources.
All those factors increase the common practice to introduce non-native medicinal plants in countries, with the purpose to reproduce this source of raw material either by private/individual or governmental entities for medical/commercial purposes. However, this intention to cultivate non-native medicinal plants will not always lead to the exact reproduction of the bioactive properties that characterize them in their natural habitat [4]. Intrinsic factors can be better controlled by determining the identity of the plant, as well as its genetic authenticity, but the external factors, such as environment, cultivation, harvest, and post-harvest processing/storage practices, become more difficult to manage, especially when the activity is developed by the population [5].
Plectranthus neochilus Schltr., an aromatic and succulent species from the Lamiaceae, is one of these plants whose cultivation has spread to almost the entire world. The endemic plant from South Africa and Namibia is commonly known as ‘blue coleus’ or ‘lobster flower’ (English speakers), ‘muskietbossie’ by Africans, and ‘boldo-rasteiro’ by Portuguese. It is traditionally used in African and Brazilian folk medicine to treat skin diseases, respiratory ailments, disturbed digestion, hepatic insufficiency, and dyspepsia [6][7]. From the pharmacologic point of view, antioxidant [8], antibacterial [9], cytotoxic [10], and α-glucosidase-inhibitory activities [11] of P. neochilus have been reported, but prevailing is the antimicrobial activity. In a recent paper, it was demonstrated that even after being used for phytoremediation, the essential oils from this plant conserves its high antimicrobial profile [12].
A few years ago, P. neochilus was introduced in Cuba and quickly started to be cultivated because of its ethnobotanical and pharmacological benefits and its ornamental properties [13]. Recently, an ethnobotanical survey revealed that this plant is mainly consumed by the Cuban population for its sedative and hypnotic effects rather than for its traditional antimicrobial and antidiabetic properties [14]. Due to the recent introduction, its wide use, and the lack of information other than ethnobotany on this species in Cuba, the purpose of this research was to investigate the pharmacognostic parameters, the phytochemical profile of leaves and extracts of P. neochilus cultivated in Cuba, as well as its antimicrobial activity.
2. Plant Quality Control Parameters
2.1. Fresh Leaves
Macroscopic determinations did not reveal phenotypical differences throughout the year of study (four lots collected) and are in agreement with the deposited botanical information for this plant in Cuba [13] and Brazil [15]. Leaves with creased edges, perinervic venation, petiole with a wedge-shaped base, membranous texture, greenish-gray color, and a strong and characteristic aromatic odor are the most representative macroscopic quality control parameters. Other remarkable characteristics are an adaxial and abaxial pubescent leaf surface, with generally short hairs. In spite of the strong and characteristic aromatic odor, none of the eight determined essential oil (EO) yields (two replicates for the four lots) allows us to obtain quantifiable values greater than the sensitivity of the determination method. Accordingly, the EO yield values (EOY) measured did not exceed the detection limit (EOY < 0.01%). In general, the EO is detected at relatively low yields. For the species that grows in Brazil, values around 0.03% have been regularly reported [6][16], while for the species that grow in Portugal [8] and South Africa, yields exceed 0.2% [17].
The micro-morphological analysis revealed that both leaf surfaces are covered with trichomes and abundant orange-colored glandular cells that are more evident in the adaxial surface when observed under the stereo-microscope 40× (Figure 1A). The multicellular and uni-serial nature of non-glandular trichomes (Figure 1B), as well as the orange-colored glandular cells (Figure 1C), can be better observed when using higher magnifications of the bright field microscope at 100×. The adaxial surface has a cuticle of approximately 1 μm thick with a single layer epidermis with polygonal cells of 10 μm in size, periclinal and anticline walls, tracing straight or convex lines that are up to 1.25 µm thick (Figure 1D). The abaxial epidermis is also 1.25 µm thick without a cuticle and has diacytic stomata (Figure 1E) with a density of 22.5/100 µm2. The parenchyma is homogeneous, lacunar, with five to six strata of amorphous cells reaching a thickness of 35 µm. Most of these observations match with the micro-morphological characteristics described for the species, which were recently updated, with the only exception of the presence of glandular trichomes [18].
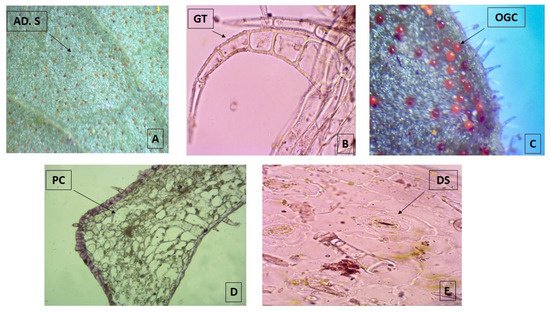
Figure 1. Photomicrograph of transverse sections of leaves from Plectranthus neochilus collected in Santiago de Cuba. (A) Adaxial surface of the foliar lamina. (B) Non-glandular trichomes. (C) Orange-colored glandular cells. (D) Polygonal cells. (E) Diacytic stomata. (A) × 40× and (B–E) × 100×.
2.2. Dry Leaves
The drying process was carried out by sun-drying, shade-drying, and oven-drying (45 °C). Due to the succulent characteristics, none of the air-exposure methods was effective since more than three weeks were needed to achieve drying, causing microbiological contamination in the case of shade-dried batches. Under these circumstances, only the oven-dried method was able to eliminate the water from the leaves in 6–8 days, preserving most of the organoleptic characteristics. The residual moisture content was 10.5 ± 1.7% (azeotropic method) (n = 8, two determinations on the four batches).
The other quality control parameters repeatable within batches (Table 1) showed no appreciable differences between the different harvesting moments. In all cases, it was possible to define an upper and lower decision limit, allowing to predict a range in which the results should be expected. Even when no statistical differences (p > 0.05) among batches were found for none of the quality control parameters, the batch selected to prepare the extracts from P. neochilus leaves was batch 2 (May), considering the maximal number of soluble compounds.
Table 1. Quantitative quality control parameters of dried leaves from Plectranthus neochilus collected in Santiago de Cuba at different times.
| Parameter | Batch 1 (February) |
Batch 2 (May) |
Batch 3 (August) |
Batch 4 (November) |
LDL (95%) | UDL (95%) |
|---|---|---|---|---|---|---|
| Total ash content (%) | 8.1 a ± 1.4 | 8.4 a ± 2.0 | 9.7 a ± 1.3 | 8.5 a ± 1.7 | 4.8 | 12.5 |
| Ethanol total soluble substances (%) | 18.5 b ± 2.0 | 20.2 b ± 2.7 | 17.5 b ± 1.9 | 20.2 b ± 0.4 | 14.5 | 23.7 |
| Water total soluble substances (%) | 22.6 c ± 1.3 | 24.1 c ± 1.4 | 22.1 c ± 1.1 | 23.5 c ± 1.6 | 19.8 | 26.3 |
LDL; Lower Decision limit, UDL; Upper Decision limit. Equal letters within the same row indicate no statistical differences (LSD Tukey test, α = 0.05).
3. Plant Extracts and Quality Control Determinations
3.1. Physical and Physicochemical Parameters of Plant and Extracts
Three extracts were prepared: the first following the ethnobotanical information [14] using water and fresh, crushed leaves (FLD). The other two extracts used dried leaves and water (DLW) or commercial ethanol at 94% (DLE) as solvents. All three extracts were prepared using the leaf material coming from batch 2. Table 2 summarizes the physical and physicochemical parameters of the extracts (three replicates).
Table 2. Physical and physicochemical parameters of the extracts prepared from batch 2 (May 2018) of Plectranthus neochilus leaves grown in Santiago de Cuba.
| Parameter | Fresh Leaves Decoction (FLD) |
Dry Leaves Water Maceration (DLW) | Dry Leaves Ethanol Maceration (DLE) |
|---|---|---|---|
| Organoleptic characteristics | Color: light green Smell: characteristic of the plant Texture: slightly dense |
Color: light brown Smell: characteristic of the plant Texture: slightly turbid |
Color: dark green Smell: characteristic of the plant Texture: transparent |
| Total extractable substances (%) | 15.99 b ± 0.01 | 20.19 c ± 0.01 | 10.67 a ± 0.01 |
| pH | 5.27 c ± 0.01 | 4.85 b ± 0.02 | 4.12 a ± 0.01 |
Different letters within the same row indicate statistical differences (LSD Tukey test, α = 0.05).
As can be seen in Table 2, the quality control parameters are different in all extracts, especially those of a quantitative character. Water stands out as a solvent with a high capacity to extract constituents of P. neochilus leaves. In relation to pH, dried leaves favor the extraction of acidic compounds.
3.2. Determination of the Phytochemical Profile of the Extracts
The three extracts of P. neochilus FLD, DLW, and DLE were analyzed by UPLC-DAD-MS/MS. Visual evaluation of the total ion chromatogram (TIC) of all extracts (Figure 2) revealed a resemblance between the extracts with regard to the position of peaks but displayed a difference in relation to the relative amount of the compounds present. This was confirmed by the dereplication analysis performed and in-depth exploration of the main peaks in the three chromatograms, which reveals that the major changes observed are related to changes in relative intensity of the peaks.
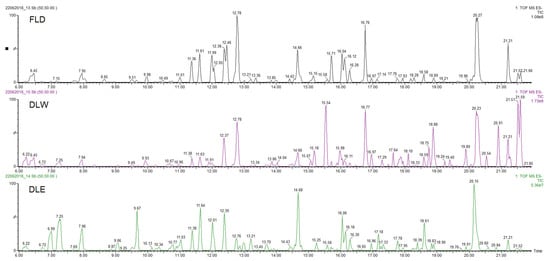
Figure 2. UPLC-DAD-MS/MS Total Ion Chromatogram (TIC) profiles of Plectranthus neochilus Schltr. extracts.
Dereplication is a strategy that provides fast identification of known metabolites in complex biological mixtures, speeding up the process to identify natural products [19], while Feature-Based Molecular Networking (FBMN), available on the Global Natural Products Social Molecular Networking (GNPS) web platform, supports the analysis of the LC-MS/MS data. This process rendered 22 library hits. The matched compounds were mainly glycosides, glucuronides, and methoxy derivates of quercetin, kaempferol, luteolin, and apigenin; abietane-type diterpenoids, fatty acyl derivates, and rosmarinic acid; helping the further process of compound identification.
Considering that the aim of this research is to evaluate why Cuban cultivars are not typically used for their potential antimicrobial effects (most reported worldwide activity), the chemical metabolite identification was focused on the extract obtained by the method that most closely resembles the inhabitants’ way of preparation (FLD). Peaks with at least 20% of relative intensity were considered, and their presence was evaluated in the other two studied extracts (DLW and DLE). Under these conditions, 18 peaks were selected and tentatively identified (Table 3, Figure 2).
Table 3. Assigned compounds, [M − H]− and ESI negative fragment ions of the eighteen peaks detected in Plectranthus neochilus fresh leaves decoction (FLD) extract.
| Compound | Rt (min) | Accurate Mass [M − H]− (m/z) | Error (ppm) | MS/MS Ions (Rel. Intensity, %) |
Molecular Formula | Tentative Identification | AEP |
|---|---|---|---|---|---|---|---|
| 1 | 6.45 | 387.1647 | −1.3 | 207(17), 163(8) | C18H28O9 | 12-Hydroxyjasmonic acid glucoside | DLW |
| 2 | 7.95 | 593.1553 | 1.5 | 503(12), 473(37), 413(5), 383(11), 353(19) | C27H30O15 | Vicenin-2 | DLW, DLE |
| 3 | 11.36 | 491.0858 | −1.0 | 475(51), 315(59), 299(64) | C22H20O13 | 4′-Methoxy-quercetin-3-O-glucuronide | DLW, DLE |
| 4 | 11.61 | 461.0721 | −0.9 | 285(57), 255(22) | C21H18O12 | Luteolin-O-glucuronide | DLW, DLE |
| 5 | 11.99 | 491.0829 | 0.4 | 315(69), 299(33) | C22H20O13 | 7-Methoxy-quercetin-3-O-glucuronide | DLW, DLE * |
| 6 | 12.05 | 437.1805 | 0.7 | 377(100), 359(86), 341(22), 331(30), 315(62) | C22H30O9 | 3,6,7,12,16-Pentahydroxy-2-acetyl-5,8,12-abietatrien-11,14-dione | DLW *, DLE * |
| 7 | 12.38 | 437.1816 | −0.2 | 377(38), 359(41), 289(71) | C22H30O9 | 2,3,7,12,16-Pentahydroxy-6-acetyl-5,8,12-abietatrien-11,14-dion | DLW, DLE |
| 8 | 12.46 | 467.2131 | 0.4 | 437(18), 421(36), 289(100) | C20H36O12 | 2-(8-(Hydroxymethoxy)oct-1-en-3-yloxy)-hexoside-pentose | None |
| 9 | 12.79 | 359.0778 | 1.6 | 197(25), 179(23), 161(48), 135(7) | C18H15O8 | Rosmarinic acid | DLW, DLE |
| 10 | 14.66 | 489.1032 | −1.4 | 313(57), 298(19), 283(18) | C23H22O12 | 3′,4′-Dimethoxy-luteolin-7-glucuronide | DLW, DLE |
| 11 | 15.71 | 479.1918 | −1.5 | 419(86), 401(62), 359(41), 341(24), 313(21) | C24H32O10 | 6,11,12,14,16-Pentahydroxy-3,17diacetyl-8,11,13-abietatrien-7-one | DLW *, DLE * |
| 12 | 16.04 | 475.0871 | −1.3 | 299(73), 284(31) | C22H20O12 | Methoxy-kaempferol-7-glucuronide | DLW *, DLE |
| 13 | 16.12 | 475.0874 | −0.6 | 299(100), 284(39) | C22H20O12 | Methoxy-kaempferol-3-glucuronide | DLW * |
| 14 | 16.28 | 459.0930 | −0.4 | 283(100), 268(51) | C22H20O11 | Methoxy-apigenin-5-glucuronide | DLW, DLE |
| 15 | 16.76 | 511.2578 | 1.0 | 493(27), 467(76), 305(9) | C26H40O10 | Hexosyl-6β-hydroxicarnosol | DLW |
| 16 | 18.58 | 435.1661 | 1.1 | 375(42), 357(19),327(9) | C22H28O9 | 3,6,11,12,14-Pentahydroxy-2-acetyl-5,7,11,13-abietatetraen-7-one | DLW, DLE |
| 17 | 20.27 | 477.1798 | −1.0 | 417(100), 387(17), 357(23), 327(11) | C24H30O10 | 6,11,12,14,16-Pentahydroxy-3,17-diacetyl-5,8,11,13-abietatetraen-7-one | DLW |
| 18 | 21.21 | 419.1721 | −1.4 | 359(51), 341(6) | C22H28O8 | 3,6,12-Trihydroxy-2-acetyl-8,12-abietadien-7,11,14-trione | DLW, DLE |
Rt→Retention Time, AEP→Peak presence in alternative extract (DLW and DLE), *→Traces.
Compound 1, with a retention time of 6.45 min, shows a peak with m/z 387.1647 [M − H]− yielding a fragment at m/z 207 [M-H-C6H12O6]-due to the loss of a neutral hexoside residue (180 Da) and another at m/z 163 [M-H-C6H12O6-COO-]- as result of the loss of the carboxyl function.
Compounds from 2 to 5, as well as 10, 12, 13, and 14, were identified as flavonoid (flavone and flavonol type) derivates in which the nature of the aglycone was inferred with the help of the MS2 ESI positive mode [20]. Compound 2 was identified as vicenin-2. Figure 3 shows the fragmentation pattern proposed assigning those product ions as a result of the cross-ring cleavages in di-hexose C-flavonoid glycoside [20][21]. The UV spectra confirm the flavonoid nature of compound 2 with 225, 270, and 375 nm peaks.
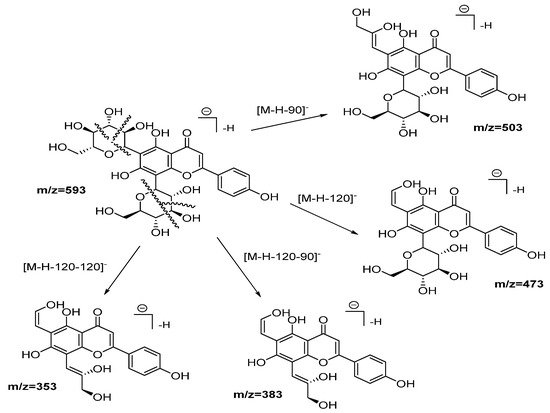
Figure 3. Fragmentation pathways (ESI negative mode) proposed for Vicenin 2.
Compounds 3 and 5 were classified as position isomers, showing similar molecular ions and fragments consistent with glucuronide loss (176 Da). As a unique difference, peak 3 displays an extra fragment at m/z 476 with relatively high intensity (51%), suggesting an easier loss of a methyl group from an ether moiety than peak 5 that, on the contrary, shows the fragment m/z 299 with higher intensity. For compound 3, a peak at 153.0118 (ESI positive mode) confirms that the –OCH3 moiety is not present in the A-ring. Those observations, together with the information derived from the FBMN analysis, and the chemotaxonomic information for the genus Plectranthus [22], allows us to assign position 4′ and 7 for the methoxy groups of compounds 3 and 5, respectively, while the glucuronide group was placed at position 3.
Compounds 6 and 7 also have almost the same molecular ions and similar initial fragmentation patterns representing the loss of an acetyl group (−60 Da) and a water molecule (−18 Da). The difference between both compounds is defined by the rest of the fragments and their relative intensity. Considering the results of dereplication analysis and the abundance of reports of abietane diterpenoids for Plectranthus species [23][24], Figure 4 is proposed as fragmentation pathways for compounds 6 and 7. The position of the hydroxyl and acetyl substituents are conditioned to the cleavage of the ring A (-C(CH3)2-CO, m/z = −70 Da), which is only possible in compound 7 after the loss of the acetyl group of position 2.
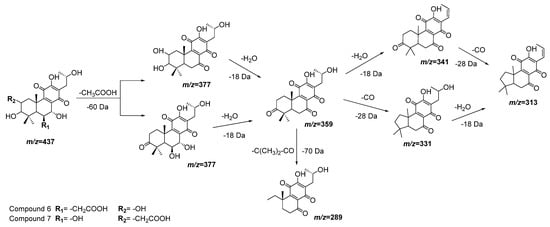
Figure 4. Fragmentation pathways (ESI negative mode) proposed for compounds 6 and 7 in Plectranthus neochilus extracts.
Compound 8 was coincident with a fractionation pattern of a fatty acyl glycoside and tentatively identified as 2-(8-(hydroxymethoxy)oct-1-en-3-yloxy)-hexoside-pentose. Fragment ions at m/z 437 and 421 are in correspondence with the cleavage on both sides of the ether bond of the aliphatic chain. The base fragment (100% intensity) at m/z 289 is a consequence of the further loss of the pentoside unit.
Compound 9 and its fragmentation pattern allowed us to identify this peak as the well-known compound rosmarinic acid. Both of the fragment ions at m/z 197 and 179 correspond to the cleavage of the ester group, while fragment m/z 161 corresponds to the loss of two or one water molecules of each one of the previous fragments, respectively. The last fragment observed, m/z 135 (even in a very low intensity), confirms the assigned substance. Based on the peak area at UV detection, this compound classifies as the main compound in this extract. This result is in concordance with previous information [25].
Compounds 10, 12, 13, and 14 are the last flavonoids identified. All are methoxy-glucuronide-flavonoid derivates. Typical for all compounds is the loss of the glucuronyl group generating the fragments at m/z 313, 299, 299, and 283, respectively (Table 3). In the case of the first two, this fragment results in the second most abundant, while for compounds 13 and 14, the fragments m/z 299 and 283, respectively, represent the main peak in the spectrum (see Figure 5). Further fragments are in agreement with the loss of methyl groups from the methyl ether (−15 Da).
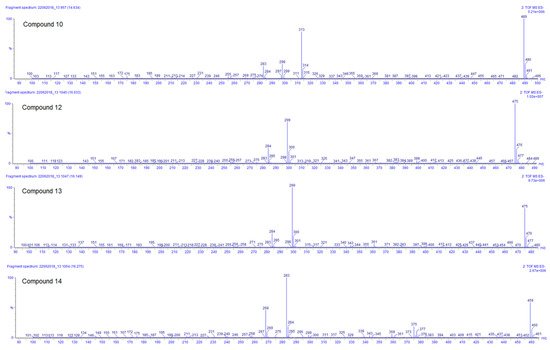
Figure 5. ESI negative ion mode MS2 spectra of compounds 10, 12, 13, and 14 of Plectranthus neochilus extracts.
For compound 10, the ESI positive mode shows a distinctive fragment at 179 (0,4B+), characteristic of the C–C cleavage at positions 0/4 of luteolin [20]. Considering all those facts, the chemotaxonomic information available, and the relative intensity of the [M − H]−, it is suggested that the glucuronyl group is attached at position 7, while methoxy groups are placed at 3′ and 4′ [20][26]. The ESI positive mode of compound 12 shows fragments at m/z 121 (0,2B+) and 165 (0,2A+), characteristic of the C–C cleavage at positions 0/2 of kaempferol [20], the rest of the factors are similar to compound 10. Compound 13 has almost the same MS and UV characteristics to compound 12. The only remarkable difference between compounds 13 and 12 is related to the relative intensity of the mass peaks. For compound 13 the main fragment was found at m/z 299, resulting from the loss of the glucuronyl, compared to compound 12 where the pseudomolecular ion [M − H]– was seen as the main ion in the spectrum (see Figure 5). This allowed us to infer that in flavonoid 13 the glucuronyl substituent is placed in the ‘non-favored’, position 3 [26]. At last, compound 14 corresponds to an apigenin derivate considering the fragment in ESI positive mode of m/z 163 (0,4B+). The main fragment (m/z 283) does not correspond to the pseudomolecular ion [M − H] –; therefore, the glucuronyl substituent was linked to the ‘less favored’ apigenin hydroxyl substituent position 5 [26]. The methoxy group was arbitrarily placed in position 7 or 4′.
Compound 11, with a pseudomolecular ion at m/z 479.1918 [M − H] – and molecular formula C24H32O10, was inferred as a diacetylditerpenoid. The consecutive loss of both acetyl fragments followed by the loss of a water molecule and the similarity with coleon D and coleon U compounds isolated from P. barbatus, P. fasciculatus, P. forsteri, P. grandidentatus, P. madagascariensis, P. nummularius, P. sanguineus, P. argentatus, and P. myrianthus [27], allows us to identify compound 11 as 6,11,12,14,18-pentahydroxy-3,17diacetyl-8,11,13-triene-7-one (Figure 6).
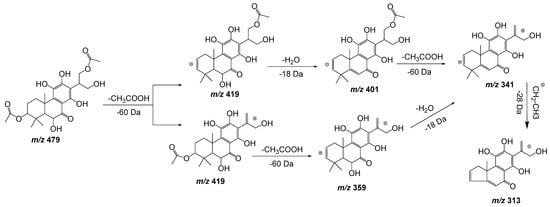
Figure 6. Fragmentation pathway (ESI negative mode) proposed for compound 11: 6,11,12,14,18-pentahydroxy-3,17diacetyl-8,11,13-triene-7-one.
Compound 15 fragments suggest a water molecule (18 Da) trans-elimination followed by the loss of a carboxyl group (44 Da), respectively. The additional loss of 162 Da indicates the presence of a hexose unit. This fragmentation pattern, the FBMN analysis, and the chemotaxonomic information available for Plectranthus genus allow us to tentatively identify compound 15 as hexosyl-6β-hydroxicarnosol. The aglycone 6β-hydroxicarnosol has already been reported for Plectranthus barbatus [28]. The unfavored loss of the hexosyl group is associated to the steric hindrance provoked by 7,20-epoxyabieta-20-one moiety, being consistent with the β position.
Compound 16 seems to have a similar backbone as compounds 6 and 7 but with an additional double bond. The fragments suggest the same sequence of fractionation with the loss of H2O (−18 Da), CH3COOH (−60 Da), H2O (−18 Da), and at last CO (−28 Da). This information allows us to identify compound 16 as 3,6,11,12,14-pentahydroxy-2-acetyl- 5,7,11,13-abietatetraen-7-one. Similar compounds have been isolated from P. madagascariensis [27] and P. scutellarioides [24].
Compound 17 shows a pseudomolecular ion at m/z 477.1798 [M − H] – corresponding to the molecular formula C24H30O10, being similar to compound 11 but with an extra double bond. Figure 7 shows the fragmentation pathway proposed for this compound. This metabolite has been previously isolated from P. scutellarioides [24].
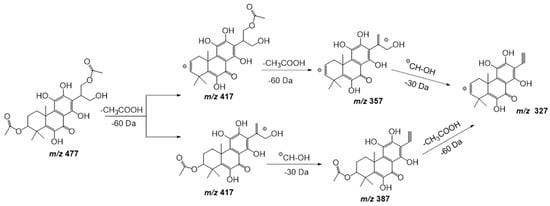
Figure 7. Fragmentation pathway (ESI negative mode) proposed for compound 17: 6,11,12,14,16-pentahydroxy-3,17diacetyl-5,8,11,13-tetraene-7-one.
The last compound identified seems to have a similar abietane backbone as compounds 6 and 7, as well as compound 17. The fragment at m/z 359 (second most abundant with 51%) can be explained by the loss of a neutral acetyl group [M-H-60] – with the consequent oxidation of the adjacent hydroxyl group at position 3, as described before with compound 7 (see Figure 4). The fragment, with a very low relative intensity (6%) at m/z 341, represents the loss of a water molecule. Based on this information, the hydroxyl group should be positioned at place 6, generating a double bond. The other hydroxyl substituent was placed in a position that does not allow easy water loss, position 12 (as chemotaxonomic pattern in most of the abietane diterpenoids identified in Plectranthus genus). All this information was also supported by the FBMN analysis. The coleon U-quinone nucleus suggested for compound 18 has been recurrently reported for Plectranthus species, such as P. madagascariensis, P. sanguineus, P. forsterii, P. grandidentatus, and P. myrianthus [27].
4. Antimicrobial Activity
The antimicrobial activity was evaluated against a wide panel of microorganisms, consisting of two bacteria (Staphylococcus aureus and Escherichia coli), one yeast (Candida albicans), one fungus (Aspergillus fumigatus), and five parasites (Leishmania infantum, Leishmania amazonensis, Trypanosoma cruzi, Trypanosoma brucei brucei, and Trypanosoma brucei rhodesiense). Neither aqueous extract (FLD and DLW) showed any significant antimicrobial activity, in agreement with the Cuban ethnobotanic information [14], but in contrast to other international reports [6][9]. The ethanol extract of the dried leaves (DLE) is the only extract with good activity against Trypanosoma species (Table 4). None of the microorganisms was sensitive to any of the three extract preparations.
Table 4. Antimicrobial activity of extracts from Plectranthus neochilus leaves growing in Santiago de Cuba.
| Extract | IC50 ± SD (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| S. aureus | E. coli | C. albicans | A. fumigatus | T. cruzi | T. b. brucei | T. b. rhodesiense | L. infantum | L. amazonensis | |
| FLD | >128 | >128 | 70.7 ± 2.4 | >128 | >128 | >128 | 64.4 ± 1.2 | >128 | >128 |
| DLW | >128 | >128 | >128 | >128 | >128 | >128 | >128 | 60.1 ± 3.2 | >128 |
| DLE | 56.6 ± 1.8 | >128 | >128 | >128 | 16.6 ± 0.6 | 16.6 ± 1.0 | 16.1 ± 0.5 | 64.9 ± 2.1 | >128 |
| Doxycycline | 0.04 ± 0.0 | 0.6 ± 0.0 | - | - | - | - | - | - | - |
| Flucytosine | - | - | 0.7 ± 0.0 | - | - | - | - | - | - |
| Terbinafine | - | - | - | 0.3 ± 0.0 | - | - | - | - | - |
| Benznidazol | - | - | - | - | 1.8 ± 0.0 | - | - | - | - |
| Suramine | - | - | - | - | - | 0.02 ± 0.0 | 0.03 ± 0.0 | - | - |
| Miltefosine | - | - | - | - | - | - | - | 10.8 ± 1.3 | |
| Glucantime | - | - | - | - | - | - | - | - | 12.3 ± 2.9 |
5. Cytotoxicity
Three different cell lines were used to test the influence of the extracts on cell viability: human fetal lung fibroblasts MRC-5SV2, murine macrophage RAW 264.7, and human monocytic THP-1cells to evaluate the safety and/or toxic action of P. neochilus extracts. The results showed that for the three cell lines, the most toxic extract (DLE) is also non-selective (Table 5). On the other hand, the aqueous extracts do not affect cell viability at the maximum concentration tested. Therefore, when combining this result with the moderate activity observed on some of the parasites, these extracts are classified as moderately selective (Table 5).
Table 5. Cytotoxicity and Selectivity index (SI) of extracts from Plectranthus neochilus leaves growing in Santiago de Cuba.
| Extract | IC50 ± SD (µg/mL) | SI | ||
|---|---|---|---|---|
| MRC-5 | RAW 264.7 | THP-1 | ||
| FLD | >256 | >256 | >256 | ≈4 (C. albicans and T. brucei rhodesiense) |
| DLW | >256 | >256 | >256 | ≈4 (L. infantum) |
| DLE | <16 | <16 | <32 | ≈1 |
| Tamoxifen | 8.3 ± 1.1 | 10.9 ± 2.3 | 10.3 ± 2.1 | - |
References
- World Health Organization Access to Medicines: Making Market Forces Serve the Poor. Ten Years in Public Health 2007–2017. 2017. Available online: https://www.who.int/publications/10-year-review/chapter-medicines.pdf?ua=1 (accessed on 3 January 2022).
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803.
- Vasisht, K.; Sharma, N.; Karan, M. Current perspective in the international trade of medicinal plants material: An update. Curr. Pharm. Des. 2016, 22, 4288–4336.
- Borges, C.V.; Minatel, I.O.; Gomez-Gomez, H.A.; Lima, G.P.P. Medicinal plants: Influence of environmental factors on the content of secondary metabolites. In Medicinal Plants and Environmental Challenges; Ghorbanpour, M., Varma, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 259–277. ISBN 978-3-319-68716-2.
- Yuan, Y.; Tang, X.; Jia, Z.; Li, C.; Ma, J.; Zhang, J. The effects of ecological factors on the main medicinal components of Dendrobium officinale under different cultivation modes. Forests 2020, 11, 94.
- Caixeta, S.C.; Magalhães, L.G.; de Melo, N.I.; Wakabayashi, K.A.L.; Aguiar, G.P.; Aguiar, D.P.; Mantovani, A.L.L.; Alves, J.M.; Oliveira, P.F.; Tavares, D.C.; et al. Chemical composition and in vitro schistosomicidal activity of the essential oil of Plectranthus neochilus grown in southeast Brazil. Chem. Biodivers. 2011, 8, 2149–2157.
- York, T.; De Wet, H.; van Vuuren, S.F. Plants used for treating respiratory infections in rural Maputaland, KwaZulu-Natal, South Africa. J. Ethnopharmacol. 2011, 135, 696–710.
- Mota, L.; Figueiredo, A.C.; Pedro, L.G.; Barroso, J.G.; Miguel, M.G.; Faleiro, M.L.; Ascensão, L. Volatile-oils composition, and bioactivity of the essential oils of Plectranthus barbatus, P. neochilus, and P. ornatus grown in Portugal. Chem. Biodivers. 2014, 11, 719–732.
- Pereira, M.; Matias, D.; Pereira, F.; Reis, C.P.; Simões, M.F.; Rijo, P. Antimicrobial screening of Plectranthus madagascariensis and P. neochilus extracts. Biomed. Biopharm. Res. 2015, 12, 127–138.
- Borges, G.A.; Ferreira, J.F.; Elias, S.T.; Guerra, E.N.S.; Silveira, D.; Simeoni, L.A. Cytotoxic effect of Plectranthus neochilus extracts in head and neck carcinoma cell lines. Afr. J. Pharm. Pharmacol. 2016, 10, 157–163.
- de Souza, P.M.; de Sales, P.M.; Simeoni, L.A.; Silva, E.C.; Silveira, D.; de Oliveira Magalhães, P. Inhibitory activity of α-amylase and α-glucosidase by plant extracts from the Brazilian cerrado. Planta Med. 2012, 78, 393–399.
- Ramborger, B.P.; Gomes Paz, M.E.; Kieling, K.M.C.; Sigal Carriço, M.R.; de Paula Gollino, G.; Costa, M.T.; Ribeiro, V.B.; Folmer, V.; Gasparotto Denardin, E.L.; de Jesus Soares, J.; et al. Toxicological parameters of aqueous residue after using Plectranthus neochilus for 2,4-D phytoremediation. Chemosphere 2021, 270, 128638.
- Mendez, S.I.E.; Rifá, T.J. Dos especies de Plectranthus (Lamiaceae) de reciente introducción en Cuba. Bouteloua 2016, 26, 92–96.
- Rodríguez-Ferreiro, A.O.; Léon-Duharte, D.; Polanco-Durán, G.; Guisado-Bourzac, F.; Ochoa-Pacheco, A.; Escalona-Arranz, J.C. Ethnobotany of Plectranthus neochilus Schltr (Meprobamate) in Cuba. Boletín Latinoam. y del Caribe de Plantas Med. y Aromáticas 2020, 19, 236–246.
- do Duarte, M.R.; Lopes, J.F. Stem and leaf anatomy of Plectranthus neochilus Schltr., Lamiaceae. Rev. Bras. Farmacogn. 2007, 17, 549–556.
- Crevelin, E.J.; Caixeta, S.C.; Dias, H.J.; Groppo, M.; Cunha, W.R.; Martins, C.H.G.; Crotti, A.E.M. Antimicrobial activity of the essential oil of Plectranthus neochilus against cariogenic bacteria. Evid.-Based Complement. Altern. Med. 2015, 2015, 102317.
- Lawal, O.A.; Hutchings, A.H.; Oyedeji, O. Chemical composition of the leaf oil of Plectranthus neochilus Schltr. J. Essent. Oil Res. 2010, 22, 546–547.
- Galbiatti, M.I.; Cassola, F.; Mesquita, A.T.; Pinheiro, G.P.; Mayer, J.L.S.; Sawaya, A.C.H.F. Plectranthus neochilus Schltr.: Anatomic and cytogenetic analyses and chemical characterization of its essential oil. S. Afr. J. Bot. 2021, 143, 97–106.
- de Oliveira, G.G.; Carnevale Neto, F.; Demarque, D.P.; de Sousa Pereira-Junior, J.A.; Sampaio Peixoto Filho, R.C.; de Melo, S.J.; da Silva Almeida, J.R.G.; Lopes, J.L.C.; Lopes, N.P. Dereplication of flavonoid glycoconjugates from Adenocalymma imperatoris-maximilianii by untargeted tandem mass spectrometry-based molecular networking. Planta Med. 2017, 83, 636–646.
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15.
- Vukics, V.; Guttman, A. Structural characterization of flavonoid glycosides by multi-stage mass spectrometry. Mass Spectrom. Rev. 2008, 29, 1–16.
- Grayer, R.J.; Eckert, M.R.; Lever, A.; Veitch, N.C.; Kite, G.C.; Paton, A.J. Distribution of exudate flavonoids in the genus Plectranthus. Biochem. Syst. Ecol. 2010, 38, 335–341.
- Abdissa, N.; Frese, M.; Sewald, N. Antimicrobial abietane-type diterpenoids from Plectranthus punctatus. Molecules 2017, 22, 1919.
- Cretton, S.; Saraux, N.; Monteillier, A.; Righi, D.; Marcourt, L.; Genta-Jouve, G.; Wolfender, J.L.; Cuendet, M.; Christen, P. Anti-inflammatory and antiproliferative diterpenoids from Plectranthus scutellarioides. Phytochemistry 2018, 154, 39–46.
- Brito, E.; Gomes, E.; Falé, P.L.; Borges, C.; Pacheco, R.; Teixeira, V.; Machuqueiro, M.; Ascensão, L.; Serralheiro, M.L.M. Bioactivities of decoctions from Plectranthus species related to their traditional use on the treatment of digestive problems and alcohol intoxication. J. Ethnopharmacol. 2018, 220, 147–154.
- Ablajan, K.; Abliz, Z.; Shang, X.-Y.; He, J.-M.; Zhang, R.-P.; Shi, J.-G. Structural characterization of flavonol 3,7-di-O-glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2006, 41, 352–360.
- Garcia, C.; Teodósio, C.; Oliveira, C.; Oliveira, C.; Díaz-Lanza, A.; Reis, C.; Duarte, N.; Rijo, P. Naturally occurring Plectranthus-derived diterpenes with antitumoral activities. Curr. Pharm. Des. 2018, 24, 4207–4236.
- Alasbahi, R.H.; Melzig, M.F. Plectranthus barbatus: A review of phytochemistry, ethnobotanical uses and pharmacology part 2. Planta Med. 2010, 76, 753–765.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
18 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




