Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Robb Krumlauf | + 2536 word(s) | 2536 | 2021-09-03 07:45:31 | | | |
| 2 | Lily Guo | Meta information modification | 2536 | 2022-01-18 02:09:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Krumlauf, R. Vertebrate Hindbrain Segmentation. Encyclopedia. Available online: https://encyclopedia.pub/entry/18365 (accessed on 08 February 2026).
Krumlauf R. Vertebrate Hindbrain Segmentation. Encyclopedia. Available at: https://encyclopedia.pub/entry/18365. Accessed February 08, 2026.
Krumlauf, Robb. "Vertebrate Hindbrain Segmentation" Encyclopedia, https://encyclopedia.pub/entry/18365 (accessed February 08, 2026).
Krumlauf, R. (2022, January 17). Vertebrate Hindbrain Segmentation. In Encyclopedia. https://encyclopedia.pub/entry/18365
Krumlauf, Robb. "Vertebrate Hindbrain Segmentation." Encyclopedia. Web. 17 January, 2022.
Copy Citation
In metazoans, Hox genes are key drivers of morphogenesis. In chordates, they play important roles in patterning the antero-posterior (A-P) axis. A crucial aspect of their role in axial patterning is their collinear expression, a process thought to be linked to their response to major signaling pathways such as retinoic acid (RA) signaling. The amplification of Hox genes following major events of genome evolution can contribute to morphological diversity. In vertebrates, RA acts as a key regulator of the gene regulatory network (GRN) underlying hindbrain segmentation, which includes Hox genes.
hindbrain
segmentation
1. Introduction
In metazoans the Hox family of transcription factors (TFs) play important roles in patterning antero-posterior (A-P) identity along the body axis [1][2][3][4][5][6][7][8][9][10]. In most organisms, Hox genes are present in the genome in tightly linked chromosomal clusters, and display highly conserved features in their organization, expression, and function [10][11][12][13][14][15][16][17][18][19]. An important property of the clustered Hox genes is collinearity (Figure 1a), which refers to their highly ordered spatial and temporal patterns of expression along the A-P axis during embryogenesis [13][20][21][22]. In any given Hox cluster, the gene located on one end of the cluster, usually Hox1, is expressed in a domain that arises early, with an anterior boundary that maps in the head region, and each successive adjacent gene in the cluster is progressively expressed later and more posteriorly (e.g., Hox2 to 15) [2][10][11][12][13][18][19][23][24][25][26][27][28][29]. This spatial and temporal program of gene expression sets up precisely ordered and nested domains of the Hox TFs along the A-P axis which form a molecular code, referred to as the ‘Hox code’. This combinatorial Hox code is used to specify and pattern different regional characteristics of tissues and structures along the A-P axis [1][2][3][4][24][30][31][32].
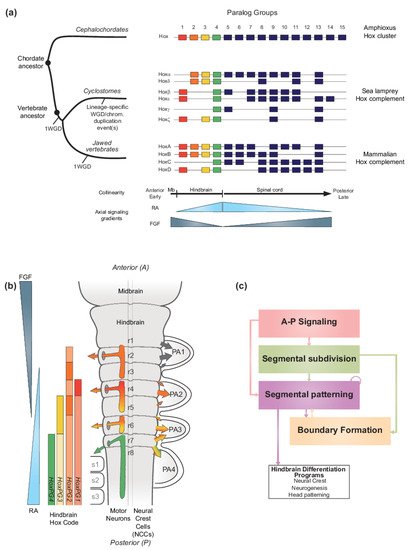
Figure 1. Hox genes and vertebrate hindbrain segmentation. (a) Schematic representation of the evolution of Hox clusters in different chordate models-amphioxus, sea lamprey and mouse, illustrating the different ways gene families can evolve following major genome rearrangements. Amphioxus displays one Hox cluster, suggesting that the chordate ancestor likely possessed one Hox cluster. In vertebrates, multiple whole genome duplication (WGD) and/or chromosomal scale duplication events, led to the amplification of the ancestral Hox cluster which is thought to have been important for generating morphological diversity. Resulting Hox complements are depicted, and paralog groups (PG) are numbered 1 to 15. HoxPG1–PG4 are color-coded to reflect their role in axial patterning of the developing brain region, including in the vertebrate hindbrain. In the early diverged jawless vertebrate group (cyclostomes), the sea lamprey Hox complement is composed of 6 clusters denoted Hoxα to ξ and Hox clusters are displayed to reflect their putative evolutionary history [33]. In contrast, in jawed vertebrates, the mammalian Hox complement is composed of 4 Hox clusters and denoted HoxA to D. The colinear property of Hox gene expression is thought to be directly linked to their response to major signaling gradients acting as morphogens such as Retinoic Acid (RA) and Fibroblast Growth Factor (FGF). In vertebrates, the colinear expression of HoxPG1-PG4 is important for the segmentation of the hindbrain. (b) Schematic representation of the segmented vertebrate hindbrain and important structures emanating from individual segments (rhombomeres (r)). In the hindbrain, Hox PG1–PG4 establish a ‘Hindbrain Hox code’ in response to RA and FGF, which strongly influences hindbrain segmentation. The Hindbrain Hox code is represented following the HoxPG1–PG4 color code, with darker shades representing higher levels of expression in specific rhombomeres. The influence of the Hox code in segmentally derived motor neurons and neural crest cells (NCCs) migrating into the pharyngeal arches (PA) is represented by colored arrows. Streams of NCCs migrating into PA1 are not influenced by the Hox code (black arrows). Panel modified from [34]. (c) Diagram representing the vertebrate hindbrain Gene Regulatory Network (GRN). The Hox code and signaling gradients constitute important aspects of a broader GRN underlying hindbrain segmentation, composed of different color-coded modules. Colored arrows indicate the regulatory relationships between different modules.
In vertebrates, the Hox code is tightly coupled to segmentation of the hindbrain (Figure 1b) [35][36][37][38][39][40] and the axial skeleton [4][32][41]. Functional studies in different species have demonstrated that, during segmentation, the Hox code sets up a molecular representation of the morphological ground plan along the A-P axis in the central nervous system (CNS) and mesoderm that regulates patterning, differentiation and wiring of hindbrain neural programs and specification of different vertebral identities [4][32][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63]. Pan-vertebrate whole genome duplication (WGD) in combination with putative lineage specific WGD and/or chromosomal duplication event(s) led to the amplification and divergence of Hox genes in the evolution of vertebrates from a single ancestral cluster at the origin of chordates (Figure 1a) [10][64][65][66][67][68][69][70][71][72][73]. As a result of at least one WGD (as discussed later), all vertebrates evolved to have four or more Hox clusters organized into 14 paralog groups. In the jawed vertebrate group, mammals have four Hox clusters while teleost fishes have 7–8 Hox clusters [74]. In the early diverged jawless vertebrate group, the cyclostomes, lampreys and hagfish have six Hox clusters (Figure 1a), indicating that additional duplication events have shaped cyclostome genomes [72][75][76]. In light of their ancient and fundamental roles in regulating morphogenesis and A-P patterning, the amplification and divergence of Hox complements in vertebrates is thought to be a driver in the emergence of new traits and evolutionary novelties by being integrated in gene regulatory networks underlying morphological diversity [2][62][77][78]
Diverse molecular and cellular mechanisms are thought to contribute to the regulation and generation of collinear domains of Hox expression [20][21][79][80][81][82][83][84][85][86]. Experimental studies in a wide range of vertebrates and invertebrates have revealed that collinear Hox expression arises in part through the ability of Hox clusters to integrate information from A-P signaling centers in response to cues from major signaling pathways, such as retinoic acid (RA), fibroblast growth factor (FGF), and Wnts [21][87][88][89][90][91][92][93]. For example, in vertebrate model systems, opposing gradients of RA and FGFs have been shown to regulate nested domains of Hox expression in the CNS and in mesodermal domains that control specification of vertebral identities and axial elongation (Figure 1a,b) [91][94][95][96][97][98][99]. In the hindbrain, RA signaling plays a key role in triggering the process of segmentation and establishes segmental patterning of Hox expression [43][91][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118][119][120][121][122][123][124]. In addition, in cultured cells differentiated into neural fates, Hox genes display temporal collinearity in response to treatment with RA [125][126][127][128][129]. This wealth of experimental data reveals a high degree of functional coupling between Hox genes and signaling pathways and suggests that this is a fundamental feature of their clustered organization which underlies the collinearity of their expression patterns.
Beyond Hox gene, evolutionary analyses have revealed that the expression domains of many genes encoding important developmental TFs and components of key signaling pathways (FGF, Hh and Wnt) are similarly aligned along the A-P axis of hemichordates and chordates [23][31][77][130][131]. This suggests that, despite very different morphologies between phyla, a deeply conserved A-P patterning system, integrating axial signaling pathways and Hox genes, may have evolved long ago in deuterostome evolution. The diversity in morphological outcomes from this ancient axial patterning system are likely to arise through differences in the downstream targets of the conserved TFs and in signals that direct the terminal differentiation programs for organogenesis and morphogenesis. Hence, the ability of vertebrate Hox clusters to coordinately respond to RA signaling in many tissues may be related to their participation in a broader and ancient regulatory mechanism that underlies the patterning of regional diversity along the A-P axis in animal development.
Understanding how Hox genes and components of the ancient A-P patterning system are regulated by signaling pathways is important for investigating the evolution and emergence of A-P patterning mechanisms in the vertebrate lineage. Indeed, vertebrates may have co-opted this ancient patterning system and coupled it to novel programs of patterning and differentiation, such as hindbrain segmentation, which may have contributed to morphological diversity. The vertebrate hindbrain is an excellent model to investigate how programs of A-P patterning are coupled to signaling gradients and to explore how changes in this coupling during evolution may be associated with the emergence of morphological diversity and complexity. In vertebrates, division of the brain into different compartments during development shows considerable variability between species, but the hindbrain is a complex coordination center that displays a remarkably high degree of conservation in all vertebrates. The hindbrain contains a sophisticated network of neural circuits that play essential roles in controlling many physiological processes and behaviors [42][43][47][49][55][56][57][58][132][133][134][135][136][137]. It also plays a central role in organization of the head and craniofacial tissues through the generation of cranial neural crest cells [138][139][140].
During embryogenesis, the basic ground plan of the hindbrain is established through a process of segmentation, which organizes the region into a series of seven transient segments named rhombomeres (r) (Figure 1b) [37][38][43][137][141][142][143][144][145][146][147][148][149][150][151][152]. Genes in the Hox1–4 paralog groups (Hox PG1–PG4) are coupled to the process of segmentation and display segmentally restricted domains of expression, resulting in a ‘hindbrain Hox code’ [36][37][115][117][149][153][154][155][156][157][158][159][160][161][162][163][164][165][166]. This code ultimately confers each rhombomere with a unique molecular identity that regulates programs of neurogenesis and elaboration of the neural circuitry associated with its distinct functions in the hindbrain [42][43][47][49][58][132][133][134][135][136][137][143][167][168]. Disruption of this code results in dramatic perturbations of hindbrain and head development [51][147][169][170][171][172]. The formation of cranial neural crest cells, whose differentiated derivatives generate most of the bone and connective tissues of the head, is also coupled to hindbrain segmentation [138]. The cranial neural crest cells delaminate and migrate from the mid/hindbrain region in an organized manner [173][174][175][176], and then differentiate to form peripheral target tissues and cranial ganglia that are in register with the segmentally organized branchiomotor and reticulospinal neurons (Figure 1b). Hence, hindbrain segmentation also makes an important contribution to global head development and craniofacial patterning.
Analyses of patterns of gene expression, phenotypes arising from mutations and perturbation of expression and characterization of cis-regulatory elements in many different vertebrate species, particularly zebrafish, Xenopus, chicken, and mouse embryos, have revealed that the regulatory basis for establishing the Hox code is embedded in a conserved gene regulatory network (GRN) underlying hindbrain segmentation [34][38][42][43][100][101][103][143][144][177][178]. For example, a detailed list of cis-regulatory modules, activities, regulatory inputs, and species of origin used for constructing this GRN can be found in Table 1 of reference [34]. This GRN may be represented or visualized as a dynamic series of progressive steps or modules associated with the respective cell and developmental processes they regulate (Figure 1c) [43][100]. This GRN also provides a framework for understanding how signaling gradients and TFs, such as RA and Hox, are integrated into regulatory circuits that form and pattern hindbrain segments with distinct A-P identities. The FGF, Wnt, and RA signaling pathways govern initial steps of the GRN for hindbrain segmentation [93] and signaling cues from these pathways act as morphogens to dictate the molecular identity and organization of cells along the A-P axis. The RA morphogen is part of a well characterized signaling pathway which is of particular importance in precisely regulating the expression of key TFs, including Hox genes, in multiple modules in the GRN.
From an evolutionary perspective, hindbrain segmentation is a trait uniquely found in the vertebrate group and is remarkably conserved in all characterized vertebrate lineages [38][43]. Hindbrain segmentation appears to be an important vertebrate innovation that, in concert with the ability to form neural crest cells, is wired into conserved GRNs for developmental programs governing head development [34][100][164][179][180]. Recent studies in jawless vertebrates, such as lamprey, have revealed that many core components and TFs in the hindbrain GRN are deeply conserved to the base of the vertebrate tree [164][179][181], however, much less is known about the level of conservation of the roles of signaling pathways in the GRN of this vertebrate group. Because of their unique position as part of an early diverged vertebrate group, jawless vertebrate models provide an important opportunity to examine whether RA played an analogous role in different aspects of the hindbrain GRN early in vertebrate evolution and how this role might have evolved.
2. RA Signaling and Vertebrate Hindbrain Segmentation
2.1. RA Signaling and Its Roles in Development
RA signaling plays important roles in many fundamental biological processes. Cues from this pathway are involved in regulation of cell growth and proliferation, differentiation, and the control of homeostasis in multiple tissues. In development, RA can act as a morphogen, i.e., a signaling molecule that forms a concentration gradient over space and time which activates target genes in a concentration-dependent manner. In early vertebrate embryogenesis, RA is involved in the regulation of heart development [182][183], body axis formation [94][113][184] and the patterning and elongation of the A-P axis, through interactions with other signaling gradients (FGF and Wnt) [94][97][185]. In addition, RA signaling provides regulatory inputs into neural differentiation programs [113][186], pancreas specification and eye, kidney, and lung development [186][187][188]. Furthermore, RA signaling has a well-established role in A-P patterning of the hindbrain, where it contributes to the dynamic regulation of the process of segmentation, which lays down a basic ground plan for the elaboration of this key coordination center in the brain [43][100][103][144][178].
The RA signaling pathway can be broken down into three general steps: (1) RA metabolism to generate active ligands, (2) RA signal transduction to modulate gene expression, and (3) RA degradation to control levels of active ligand (Figure 2a). Components of the RA signaling pathway involve a Vitamin A precursor, binding proteins that mediate its extra- and intracellular transport, metabolic enzymes that convert it to an active ligand (e.g., all-trans RA and 9-cis RA) and enzymes that control the degradation of RA (Figure 2a). Signal transduction is mediated by the interaction of RA with members of the nuclear hormone receptor family of proteins (RAR and RXR) which bind directly to DNA regulatory elements in the genome and modulate patterns of gene expression [189][190]. The synthesizing and degrading enzymes, transport proteins, receptors and DNA regulatory elements together constitute the RA machinery and provide multiple opportunities for evolving and regulating the RA signaling pathway in various biological processes [187][188]. In this section, we summarize what is known about components of the RA signaling pathway and relate it to hindbrain segmentation, Hox genes, and the evolution of A-P patterning in vertebrates. The primary focus of this review is on the canonical pathway of RA signaling. However, there is emerging evidence concerning the non-canonical functions of certain components of the pathway, such as the cytoplasmic function of RARγ in cell death [191].
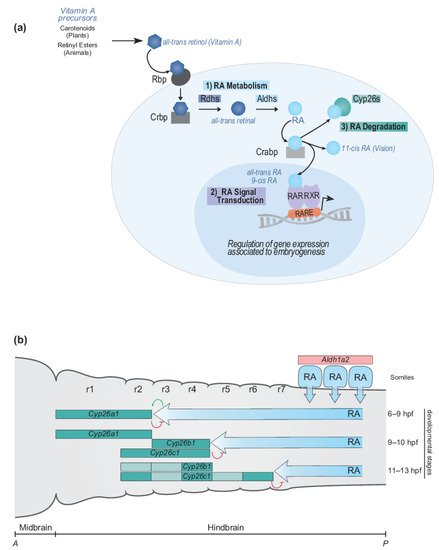
Figure 2. RA signaling pathway and RA morphogen gradient during hindbrain development. (a) Simplified schematic representation of major steps of the RA signaling pathway and main components of the RA machinery. The RA signaling pathway converts Vitamin A to RA following three important steps: (1) RA metabolism, (2) RA signal transduction and (3) RA degradation. The RA machinery includes main actors of the signaling pathway: RA synthesizing and degrading enzymes (Rdhs; Aldhs; Cyp26s), transport proteins (Rbp; Crbp; Crabp), nuclear hormone receptors (RAR-RXRs), and Retinoic Acid Response Elements (RAREs). (b) Schematic depiction of the dynamic regulation of endogenous levels of RA during hindbrain development. The RA morphogen gradient is set up by synthesizing and degrading enzymes of the RA machinery in the context of the vertebrate hindbrain. Aldh1a2 is expressed in the somites where Aldh1a2 localizes and synthesizes RA. The spatially and temporally dynamic segmental expression of Cyp26a1, Cyp26b1, and Cyp26c1 induces shifting domains of Cyp26 degrading activity, which act as anterior sinks of RA activity, therefore generating a precise and gradual set up of the RA morphogen gradient. The developmental time points correspond to zebrafish development. A, anterior; P, posterior; r, rhombomere.
References
- Carroll, S.B. Homeotic genes and the evolution of arthropods and chordates. Nature 1995, 376, 479–485.
- Martin, A.; Serano, J.M.; Jarvis, E.; Bruce, H.S.; Wang, J.; Ray, S.; Barker, C.A.; O’Connell, L.C.; Patel, N.H. CRISPR/Cas9 Mutagenesis Reveals Versatile Roles of Hox Genes in Crustacean Limb Specification and Evolution. Curr. Biol. 2016, 26, 14–26.
- McGinnis, W.; Krumlauf, R. Homeobox genes and axial patterning. Cell 1992, 68, 283–302.
- Mallo, M.; Wellik, D.M.; Deschamps, J. Hox genes and regional patterning of the vertebrate body plan. Dev. Biol. 2010, 344, 7–15.
- Diederich, R.J.; Pattatucci, A.M.; Kaufman, T.C. Developmental and evolutionary implications of labial, Deformed and engrailed expression in the Drosophila head. Development 1991, 113, 273–281.
- Lutz, B.; Lu, H.C.; Eichele, G.; Miller, D.; Kaufman, T.C. Rescue of Drosophila labial null mutant by the chicken ortholog Hoxb-1 demonstrates that the function of Hox genes is phylogenetically conserved. Genes Dev. 1996, 10, 176–184.
- Powers, T.P.; Hogan, J.; Ke, Z.; Dymbrowski, K.; Wang, X.; Collins, F.H.; Kaufman, T.C. Characterization of the Hox cluster from the mosquito Anopheles gambiae (Diptera: Culicidae). Evol. Dev. 2000, 2, 311–325.
- Hughes, C.L.; Kaufman, T.C. Exploring the myriapod body plan: Expression patterns of the ten Hox genes in a centipede. Development 2002, 129, 1225–1238.
- Singh, N.P.; De Kumar, B.; Paulson, A.; Parrish, M.E.; Zhang, Y.; Florens, L.; Conaway, J.W.; Si, K.; Krumlauf, R. A six-amino-acid motif is a major determinant in functional evolution of HOX1 proteins. Genes Dev. 2020, 34, 1680–1696.
- Ferrier, D.E.; Holland, P.W. Ancient origin of the Hox gene cluster. Nat. Rev. Genet. 2001, 2, 33–38.
- Duboule, D.; Dolle, P. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO J. 1989, 8, 1497–1505.
- Graham, A.; Papalopulu, N.; Krumlauf, R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell 1989, 57, 367–378.
- Lewis, E.B. A gene complex controlling segmentation in Drosophila. Nature 1978, 276, 565–570.
- Scott, M.P.; Weiner, A.J. Structural relationships among genes that control development: Sequence homology between the Antennapedia, Ultrabithorax and fushi tarazu loci of Drosophila. Proc. Natl. Acad. Sci. USA 1984, 81, 4115–4119.
- Scott, M.; Weiner, A.; Hazelrigg, T.; Polisky, B.; Pirotta, V.; Scalenghe, F.; Kaufman, M. The molecular organization of the Antennapedia locus of Drosophila. Cell 1983, 35, 763–776.
- McGinnis, W.; Garber, R.L.; Wirz, J.; Kuroiwa, A.; Gehring, W. A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell 1984, 37, 403–408.
- McGinnis, W.; Levine, M.S.; Hafen, E.; Kuroiwa, A.; Gehring, W. A conserved DNA sequence in homeotic genes of the Drosophila Antennapedia and bithorax complexes. Nature 1984, 308, 428–433.
- Harding, K.; Wedeen, C.; McGinnis, W.; Levine, M. Spatially regulated expression of homeotic genes in Drosophila. Science 1985, 229, 1236–1242.
- Dekker, E.J.; Pannese, M.; Houtzager, E.; Boncinelli, E.; Durston, A. Colinearity in the Xenopus laevis Hox-2 complex. Mech. Dev. 1992, 40, 3–12.
- Kmita, M.; Duboule, D. Organizing axes in time and space; 25 years of colinear tinkering. Science 2003, 301, 331–333.
- Deschamps, J.; Duboule, D. Embryonic timing, axial stem cells, chromatin dynamics, and the Hox clock. Genes Dev. 2017, 31, 1406–1416.
- Duboule, D.; Morata, G. Colinearity and functional hierarchy among genes of the homeotic complexes. Trends Genet. 1994, 10, 358–364.
- Lowe, C.J.; Wu, M.; Salic, A.; Evans, L.; Lander, E.; Stange-Thomann, N.; Gruber, C.E.; Gerhart, J.; Kirschner, M. Anteroposterior patterning in hemichordates and the origins of the chordate nervous system. Cell 2003, 113, 853–865.
- Serano, J.M.; Martin, A.; Liubicich, D.M.; Jarvis, E.; Bruce, H.S.; La, K.; Browne, W.E.; Grimwood, J.; Patel, N.H. Comprehensive analysis of Hox gene expression in the amphipod crustacean Parhyale hawaiensis. Dev. Biol. 2016, 409, 297–309.
- Bachiller, D.; Macias, A.; Duboule, D.; Morata, G. Conservation of a functional hierarchy between mammalian and insect Hox/HOM genes. EMBO J. 1994, 13, 1930–1941.
- Gaunt, S.J.; Strachan, L. Temporal colinearity in expression of anterior Hox genes in developing chick embryos. Dev. Dyn. 1996, 207, 270–280.
- Duboule, D. Temporal colinearity and phylotypic progression: A basis for the stability of a vertebrate Bauplan and the evolution of morphologies through heterochrony. Development 1994, 135–142.
- Duboule, D. Vertebrate hox gene regulation: Clustering and/or colinearity? Curr. Opin. Genet. Dev. 1998, 8, 514–518.
- Gaunt, S.J.; Sharpe, P.T.; Duboule, D. Spatially restricted domains of homeo-gene transcripts in mouse embryos: Relation to a segmented body plan. Development 1988, 104, 169–181.
- Krumlauf, R. Hox genes in vertebrate development. Cell 1994, 78, 191–201.
- Lowe, C.J.; Clarke, D.N.; Medeiros, D.M.; Rokhsar, D.S.; Gerhart, J. The deuterostome context of chordate origins. Nature 2015, 520, 456–465.
- Kessel, M.; Gruss, P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell 1991, 67, 89–104.
- Parker, H.J.; Bronner, M.E.; Krumlauf, R. An atlas of anterior hox gene expression in the embryonic sea lamprey head: Hox-code evolution in vertebrates. Dev. Biol. 2019, 453, 19–33.
- Parker, H.J.; Krumlauf, R. Segmental arithmetic: Summing up the Hox gene regulatory network for hindbrain development in chordates. Wiley Interdiscip. Rev. Dev. Biol. 2017, 6, e286.
- Wilkinson, D.G.; Bhatt, S.; Cook, M.; Boncinelli, E.; Krumlauf, R. Segmental expression of Hox-2 homoeobox-containing genes in the developing mouse hindbrain. Nature 1989, 341, 405–409.
- Hunt, P.; Gulisano, M.; Cook, M.; Sham, M.H.; Faiella, A.; Wilkinson, D.; Boncinelli, E.; Krumlauf, R. A distinct Hox code for the branchial region of the vertebrate head. Nature 1991, 353, 861–864.
- Prince, V.E.; Moens, C.B.; Kimmel, C.B.; Ho, R.K. Zebrafish hox genes: Expression in the hindbrain region of wild-type and mutants of the segmentation gene, valentino. Development 1998, 125, 393–406.
- Lumsden, A. Segmentation and compartition in the early avian hindbrain. Mech. Dev. 2004, 121, 1081–1088.
- Murphy, P.; Davidson, D.R.; Hill, R.E. Segment-specific expression of a homeobox-containing gene in the mouse hindbrain. Nature 1989, 341, 156–159.
- Murphy, P.; Hill, R.E. Expression of the mouse labial-like homeobox-containing genes, Hox 2.9 and Hox 1.6, during segmentation of the hindbrain. Development 1991, 111, 61–74.
- Kessel, M.; Balling, R.; Gruss, P. Variations of cervical vertebrae after expression of a Hox-1.1 transgene in mice. Cell 1990, 61, 301–308.
- Alexander, T.; Nolte, C.; Krumlauf, R. Hox genes and segmentation of the hindbrain and axial skeleton. Annu. Rev. Cell Dev. Biol. 2009, 25, 431–456.
- Krumlauf, R.; Wilkinson, D.G. Segmentation and patterning of the vertebrate hindbrain. Development 2021, 148, dev186460.
- Balling, R.; Mutter, G.; Gruss, P.; Kessel, M. Craniofacial abnormalities induced by ectopic expression of the homeobox gene Hox-1.1 in transgenic mice. Cell 1989, 58, 337–347.
- Zhang, M.; Kim, H.J.; Marshall, H.; Gendron-Maguire, M.; Lucas, D.A.; Baron, A.; Gudas, L.J.; Gridley, T.; Krumlauf, R.; Grippo, J.F. Ectopic Hoxa-1 induces rhombomere transformation in mouse hindbrain. Development 1994, 120, 2431–2442.
- Alexandre, D.; Clarke, J.D.; Oxtoby, E.; Yan, Y.L.; Jowett, T.; Holder, N. Ectopic expression of Hoxa-1 in the zebrafish alters the fate of the mandibular arch neural crest and phenocopies a retinoic acid-induced phenotype. Development 1996, 122, 735–746.
- Di Bonito, M.; Narita, Y.; Avallone, B.; Sequino, L.; Mancuso, M.; Andolfi, G.; Franze, A.M.; Puelles, L.; Rijli, F.M.; Studer, M. Assembly of the auditory circuitry by a Hox genetic network in the mouse brainstem. PLoS Genet. 2013, 9, e1003249.
- Geisen, M.J.; Di Meglio, T.; Pasqualetti, M.; Ducret, S.; Brunet, J.F.; Chedotal, A.; Rijli, F.M. Hox paralog group 2 genes control the migration of mouse pontine neurons through slit-robo signaling. PLoS Biol. 2008, 6, e142.
- Chatonnet, F.; Wrobel, L.J.; Mezieres, V.; Pasqualetti, M.; Ducret, S.; Taillebourg, E.; Charnay, P.; Rijli, F.M.; Champagnat, J. Distinct roles of Hoxa2 and Krox20 in the development of rhythmic neural networks controlling inspiratory depth, respiratory frequency, and jaw opening. Neural Dev. 2007, 2, 19.
- Oury, F.; Murakami, Y.; Renaud, J.S.; Pasqualetti, M.; Charnay, P.; Ren, S.Y.; Rijli, F.M. Hoxa2- and rhombomere-dependent development of the mouse facial somatosensory map. Science 2006, 313, 1408–1413.
- Studer, M.; Lumsden, A.; Ariza-McNaughton, L.; Bradley, A.; Krumlauf, R. Altered segmental identity and abnormal migration of motor neurons in mice lacking Hoxb-1. Nature 1996, 384, 630–634.
- Gavalas, A.; Ruhrberg, C.; Livet, J.; Henderson, C.E.; Krumlauf, R. Neuronal defects in the hindbrain of Hoxa1, Hoxb1 and Hoxb2 mutants reflect regulatory interactions among these Hox genes. Development 2003, 130, 5663–5679.
- Gavalas, A.; Studer, M.; Lumsden, A.; Rijli, F.M.; Krumlauf, R.; Chambon, P. Hoxa1 and Hoxb1 synergize in patterning the hindbrain, cranial nerves and second pharyngeal arch. Development 1998, 125, 1123–1136.
- Davenne, M.; Maconochie, M.K.; Neun, R.; Pattyn, A.; Chambon, P.; Krumlauf, R.; Rijli, F.M. Hoxa2 and Hoxb2 control dorsoventral patterns of neuronal development in the rostral hindbrain. Neuron 1999, 22, 677–691.
- Gaufo, G.O.; Wu, S.; Capecchi, M.R. Contribution of Hox genes to the diversity of the hindbrain sensory system. Development 2004, 131, 1259–1266.
- Gaufo, G.O.; Thomas, K.R.; Capecchi, M.R. Hox3 genes coordinate mechanisms of genetic suppression and activation in the generation of branchial and somatic motoneurons. Development 2003, 130, 5191–5201.
- Arenkiel, B.R.; Tvrdik, P.; Gaufo, G.O.; Capecchi, M.R. Hoxb1 functions in both motoneurons and in tissues of the periphery to establish and maintain the proper neuronal circuitry. Genes Dev. 2004, 18, 1539–1552.
- Briscoe, J.; Wilkinson, D.G. Establishing neuronal circuitry: Hox genes make the connection. Genes Dev. 2004, 18, 1643–1648.
- Ramirez-Solis, R.; Zheng, H.; Whiting, J.; Krumlauf, R.; Bradley, A. Hoxb-4 (Hox-2.6) mutant mice show homeotic transformation of a cervical vertebra and defects in the closure of the sternal rudiments. Cell 1993, 73, 279–294.
- Condie, B.G.; Capecchi, M.R. Mice homozygous for a targeted disruption of Hoxd-3(Hox-4.1) exhibit anterior transformations of the first and second cervical vertebrae, the atlas and axis. Development 1993, 119, 579–595.
- Kostic, D.; Capecchi, M.R. Targeted disruptions of the murine Hoxa-4 and Hoxa-6 genes result in homeotic transformations of components of the vertebral column. Mech. Dev. 1994, 46, 231–247.
- Guerreiro, I.; Nunes, A.; Woltering, J.M.; Casaca, A.; Novoa, A.; Vinagre, T.; Hunter, M.E.; Duboule, D.; Mallo, M. Role of a polymorphism in a Hox/Pax-responsive enhancer in the evolution of the vertebrate spine. Proc. Natl. Acad. Sci. USA 2013, 110, 10682–10686.
- Jungbluth, S.; Bell, E.; Lumsden, A. Specification of distinct motor neuron identities by the singular activities of individual Hox genes. Development 1999, 126, 2751–2758.
- Meyer, A.; Schartl, M. Gene and genome duplications in vertebrates: The one-to-four (-to-eight in fish) rule and the evolution of novel gene functions. Curr. Opin. Cell Biol. 1999, 11, 699–704.
- Taylor, J.S.; Braasch, I.; Frickey, T.; Meyer, A.; Van de Peer, Y. Genome duplication, a trait shared by 22,000 species of ray-finned fish. Genome Res. 2003, 13, 382–390.
- Vandepoele, K.; De Vos, W.; Taylor, J.S.; Meyer, A.; Van de Peer, Y. Major events in the genome evolution of vertebrates: Paranome age and size differ considerably between ray-finned fishes and land vertebrates. Proc. Natl. Acad. Sci. USA 2004, 101, 1638–1643.
- Siegel, N.; Hoegg, S.; Salzburger, W.; Braasch, I.; Meyer, A. Comparative genomics of ParaHox clusters of teleost fishes: Gene cluster breakup and the retention of gene sets following whole genome duplications. BMC Genom. 2007, 8, 312.
- Kuraku, S.; Meyer, A.; Kuratani, S. Timing of genome duplications relative to the origin of the vertebrates: Did cyclostomes diverge before or after? Mol. Biol. Evol. 2009, 26, 47–59.
- Holland, P.W.; Takahashi, T. The evolution of homeobox genes: Implications for the study of brain development. Brain Res. Bull. 2005, 66, 484–490.
- Holland, P.W. Evolution of homeobox genes. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 31–45.
- Smith, J.J.; Keinath, M.C. The sea lamprey meiotic map improves resolution of ancient vertebrate genome duplications. Genome Res. 2015, 25, 1081–1090.
- Smith, J.J.; Timoshevskaya, N.; Ye, C.; Holt, C.; Keinath, M.C.; Parker, H.J.; Cook, M.E.; Hess, J.E.; Narum, S.R.; Lamanna, F.; et al. The sea lamprey germline genome provides insights into programmed genome rearrangement and vertebrate evolution. Nat. Genet. 2018, 50, 270–277.
- Duboule, D. The rise and fall of Hox gene clusters. Development 2007, 134, 2549–2560.
- Kuraku, S.; Meyer, A. The evolution and maintenance of Hox gene clusters in vertebrates and the teleost-specific genome duplication. Int. J. Dev. Biol. 2009, 53, 765–773.
- Pascual-Anaya, J.; Sato, I.; Sugahara, F.; Higuchi, S.; Paps, J.; Ren, Y.; Takagi, W.; Ruiz-Villalba, A.; Ota, K.G.; Wang, W.; et al. Hagfish and lamprey Hox genes reveal conservation of temporal colinearity in vertebrates. Nat. Ecol. Evol. 2018, 2, 859–866.
- Mehta, T.K.; Ravi, V.; Yamasaki, S.; Lee, A.P.; Lian, M.M.; Tay, B.H.; Tohari, S.; Yanai, S.; Tay, A.; Brenner, S.; et al. Evidence for at least six Hox clusters in the Japanese lamprey (Lethenteron japonicum). Proc. Natl. Acad. Sci. USA 2013, 110, 16044–16049.
- Holland, P.W.H.; Garcia-Fernandez, J. Hox genes and chordate evolution. Dev. Biol. 1996, 173, 382–395.
- Shimeld, S.M.; Holland, P.W. Vertebrate innovations. Proc. Natl. Acad. Sci. USA 2000, 97, 4449–4452.
- Duboule, D.; Deschamps, J. Colinearity loops out. Dev. Cell 2004, 6, 738–740.
- Durston, A.J.; Jansen, H.J.; Wacker, S.A. Review: Time-space translation regulates trunk axial patterning in the early vertebrate embryo. Genomics 2010, 95, 250–255.
- Narendra, V.; Rocha, P.P.; An, D.; Raviram, R.; Skok, J.A.; Mazzoni, E.O.; Reinberg, D. CTCF establishes discrete functional chromatin domains at the Hox clusters during differentiation. Science 2015, 347, 1017–1021.
- Durston, A.J. Vertebrate hox temporal collinearity: Does it exist and what is it’s function? Cell Cycle 2019, 18, 523–530.
- Kmita, M.; van Der Hoeven, F.; Zakany, J.; Krumlauf, R.; Duboule, D. Mechanisms of Hox gene colinearity: Transposition of the anterior Hoxb1 gene into the posterior HoxD complex. Genes Dev. 2000, 14, 198–211.
- Ahn, Y.; Mullan, H.E.; Krumlauf, R. Long-range regulation by shared retinoic acid response elements modulates dynamic expression of posterior Hoxb genes in CNS development. Dev. Biol. 2014, 388, 134–144.
- Nolte, C.; Jinks, T.; Wang, X.; Martinez Pastor, M.T.; Krumlauf, R. Shadow enhancers flanking the HoxB cluster direct dynamic Hox expression in early heart and endoderm development. Dev. Biol. 2013, 383, 158–173.
- Kondo, T.; Duboule, D. Breaking colinearity in the mouse HoxD complex. Cell 1999, 97, 407–417.
- Darras, S.; Fritzenwanker, J.H.; Uhlinger, K.R.; Farrelly, E.; Pani, A.M.; Hurley, I.A.; Norris, R.P.; Osovitz, M.; Terasaki, M.; Wu, M.; et al. Anteroposterior axis patterning by early canonical Wnt signaling during hemichordate development. PLoS Biol. 2018, 16, e2003698.
- Neijts, R.; Amin, S.; van Rooijen, C.; Tan, S.; Creyghton, M.P.; de Laat, W.; Deschamps, J. Polarized regulatory landscape and Wnt responsiveness underlie Hox activation in embryos. Genes Dev. 2016, 30, 1937–1942.
- Pownall, M.; Tucker, A.; Slack, J.; Isaacs, H. eFGF, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis in Xenopus. Development 1996, 122, 3881–3892.
- Pownall, M.E.; Isaacs, H.V.; Slack, J.M. Two phases of Hox gene regulation during early Xenopus development. Curr. Biol. 1998, 8, 673–676.
- Bel-Vialar, S.; Itasaki, N.; Krumlauf, R. Initiating Hox gene expression: In the early chick neural tube differential sensitivity to FGF and RA signaling subdivides the HoxB genes in two distinct groups. Development 2002, 129, 5103–5115.
- In der Rieden, P.M.; Vilaspasa, F.L.; Durston, A.J. Xwnt8 directly initiates expression of labial Hox genes. Dev. Dyn. 2010, 239, 126–139.
- Frank, D.; Sela-Donenfeld, D. Hindbrain induction and patterning during early vertebrate development. Cell Mol. Life Sci. 2019, 76, 941–960.
- Deschamps, J.; van Nes, J. Developmental regulation of the Hox genes during axial morphogenesis in the mouse. Development 2005, 132, 2931–2942.
- Young, T.; Rowland, J.E.; van de Ven, C.; Bialecka, M.; Novoa, A.; Carapuco, M.; van Nes, J.; de Graaff, W.; Duluc, I.; Freund, J.N.; et al. Cdx and Hox genes differentially regulate posterior axial growth in mammalian embryos. Dev. Cell 2009, 17, 516–526.
- Diez del Corral, R.; Olivera-Martinez, I.; Goriely, A.; Gale, E.; Maden, M.; Storey, K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron 2003, 40, 65–79.
- Diez del Corral, R.; Storey, K.G. Opposing FGF and retinoid pathways: A signalling switch that controls differentiation and patterning onset in the extending vertebrate body axis. BioEssays 2004, 26, 857–869.
- Ye, Z.; Kimelman, D. Hox13 genes are required for mesoderm formation and axis elongation during early zebrafish development. Development 2020, 147, dev185298.
- Bel-Vialar, S.; Core, N.; Terranova, R.; Goudot, V.; Boned, A.; Djabali, M. Altered retinoic acid sensitivity and temporal expression of Hox genes in polycomb-M33-deficient mice. Dev. Biol. 2000, 224, 238–249.
- Parker, H.J.; Krumlauf, R. A Hox gene regulatory network for hindbrain segmentation. Curr. Top. Dev. Biol. 2020, 139, 169–203.
- White, R.J.; Schilling, T.F. How degrading: Cyp26s in hindbrain development. Dev. Dyn. 2008, 237, 2775–2790.
- Sosnik, J.; Zheng, L.; Rackauckas, C.V.; Digman, M.; Gratton, E.; Nie, Q.; Schilling, T.F. Noise modulation in retinoic acid signaling sharpens segmental boundaries of gene expression in the embryonic zebrafish hindbrain. Elife 2016, 5, e14034.
- Sirbu, I.O.; Gresh, L.; Barra, J.; Duester, G. Shifting boundaries of retinoic acid activity control hindbrain segmental gene expression. Development 2005, 132, 2611–2622.
- Begemann, G.; Meyer, A. Hindbrain patterning revisited: Timing and effects of retinoic acid signalling. BioEssays 2001, 23, 981–986.
- Durston, A.J.; Timmermans, J.P.; Hage, W.J.; Hendriks, H.F.; de Vries, N.J.; Heideveld, M.; Nieuwkoop, P.D. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature 1989, 340, 140–144.
- Godsave, S.F.; Koster, C.H.; Getahun, A.; Mathu, M.; Hooiveld, M.; van der Wees, J.; Hendriks, J.; Durston, A.J. Graded retinoid responses inthe developing hindbrain. Dev. Dyn. 1998, 213, 39–49.
- van der Wees, J.; Schilthuis, J.G.; Koster, C.H.; Diesveld-Schipper, H.; Folkers, G.E.; van der Saag, P.T.; Dawson, M.I.; Shudo, K.; van der Burg, B.; Durston, A.J. Inhibition of retinoic acid receptor-mediated signalling alters positional identity in the developing hindbrain. Development 1998, 125, 545–556.
- Gale, E.; Prince, V.; Lumsden, A.; Clarke, J.; Holder, N.; Maden, M. Late effects of retinoic acid on neural crest and aspects of rhombomere. Development 1996, 122, 783–793.
- Maden, M.; Hunt, P.; Eriksson, U.; Kuroiwa, A.; Krumlauf, R.; Summerbell, D. Retinoic acid-binding protein, rhombomeres and the neural crest. Development 1991, 111, 35–43.
- Maden, M.; Holder, N. Retinoic acid and development of the central nervous system. Bioessays 1992, 14, 431–438.
- Maden, M.; Gale, E.; Kostetskii, I.; Zile, M. Vitamin A deficient quail embryos have half a hindbrain and other neural defects. Curr. Biol. 1996, 6, 417–426.
- Gale, E.; Zile, M.; Maden, M. Hindbrain respecification in the retinoid-deficient quail. Mech. Dev. 1999, 89, 43–54.
- Maden, M. Retinoid signalling in the development of the central nervous system. Nat. Rev. Neurosci. 2002, 3, 843–853.
- Marshall, H.; Nonchev, S.; Sham, M.H.; Muchamore, I.; Lumsden, A.; Krumlauf, R. Retinoic acid alters hindbrain Hox code and induces transformation of rhombomeres 2/3 into a 4/5 identity. Nature 1992, 360, 737–741.
- Studer, M.; Popperl, H.; Marshall, H.; Kuroiwa, A.; Krumlauf, R. Role of a conserved retinoic acid response element in rhombomere restriction of Hoxb-1. Science 1994, 265, 1728–1732.
- Marshall, H.; Morrison, A.; Studer, M.; Popperl, H.; Krumlauf, R. Retinoids and Hox genes. FASEB J. 1996, 10, 969–978.
- Gould, A.; Itasaki, N.; Krumlauf, R. Initiation of rhombomeric Hoxb4 expression requires induction by somites and a retinoid pathway. Neuron 1998, 21, 39–51.
- Sundin, O.; Eichele, G. An early marker of axial pattern in the chick embryo and its respecification by retinoic acid. Development 1992, 114, 841–852.
- Dupe, V.; Lumsden, A. Hindbrain patterning involves graded responses to retinoic acid signalling. Development 2001, 128, 2199–2208.
- Lloret-Vilaspasa, F.; Jansen, H.J.; de Roos, K.; Chandraratna, R.A.; Zile, M.H.; Stern, C.D.; Durston, A.J. Retinoid signalling is required for information transfer from mesoderm to neuroectoderm during gastrulation. Int. J. Dev. Biol. 2010, 54, 599–608.
- Dekker, E.J.; Pannese, M.; Houtzager, E.; Timmermans, A.; Boncinelli, E.; Durston, A. Xenopus Hox-2 genes are expressed sequentially after the onset of gastrulation and are differentially inducible by retinoic acid. Dev. Suppl. 1992, 195–202.
- Durston, A.J. What are the roles of retinoids, other morphogens, and Hox genes in setting up the vertebrate body axis? Genesis 2019, 57, e23296.
- Papalopulu, N.; Clarke, J.D.; Bradley, L.; Wilkinson, D.; Krumlauf, R.; Holder, N. Retinoic acid causes abnormal development and segmental patterning of the anterior hindbrain in Xenopus embryos. Development 1991, 113, 1145–1158.
- Skromne, I.; Thorsen, D.; Hale, M.; Prince, V.E.; Ho, R.K. Repression of the hindbrain developmental program by Cdx factors is required for the specification of the vertebrate spinal cord. Development 2007, 134, 2147–2158.
- Simeone, A.; Acampora, D.; Arcioni, L.; Andrews, P.W.; Boncinelli, E.; Mavilio, F. Sequential activation of HOX2 homeobox genes by retinoic acid in human embryonal carcinoma cells. Nature 1990, 346, 763–766.
- Simeone, A.; Acampora, D.; Nigro, V.; Faiella, A.; D’Esposito, M.; Stornaiuolo, A.; Mavilio, F.; Boncinelli, E. Differential regulation by retinoic acid of the homeobox genes of the four HOX loci in human embryonal carcinoma cells. Mech. Dev. 1991, 33, 215–227.
- Papalopulu, N.; Lovell-Badge, R.; Krumlauf, R. The expression of murine Hox-2 genes is dependent on the differentiation pathway and displays a collinear sensitivity to retinoic acid in F9 cells and Xenopus embryos. Nucleic Acids Res. 1991, 19, 5497–5506.
- De Kumar, B.; Parrish, M.E.; Slaughter, B.D.; Unruh, J.R.; Gogol, M.; Seidel, C.; Paulson, A.; Li, H.; Gaudenz, K.; Peak, A.; et al. Analysis of dynamic changes in retinoid-induced transcription and epigenetic profiles of murine Hox clusters in ES cells. Genome Res. 2015, 25, 1229–1243.
- Mazzoni, E.O.; Mahony, S.; Peljto, M.; Patel, T.; Thornton, S.R.; McCuine, S.; Reeder, C.; Boyer, L.A.; Young, R.A.; Gifford, D.K.; et al. Saltatory remodeling of Hox chromatin in response to rostrocaudal patterning signals. Nat. Neurosci. 2013, 16, 1191–1198.
- Pani, A.M.; Mullarkey, E.E.; Aronowicz, J.; Assimacopoulos, S.; Grove, E.A.; Lowe, C.J. Ancient deuterostome origins of vertebrate brain signalling centres. Nature 2012, 483, 289–294.
- Gerhart, J.; Lowe, C.; Kirschner, M. Hemichordates and the origin of chordates. Curr. Opin. Genet. Dev. 2005, 15, 461–467.
- Kiecker, C.; Lumsden, A. Compartments and their boundaries in vertebrate brain development. Nat. Rev. Neurosci. 2005, 6, 553–564.
- Gilland, E.; Baker, R. Conservation of neuroepithelial and mesodermal segments in the embryonic vertebrate head. Acta Anat. 1993, 148, 110–123.
- Gilland, E.; Baker, R. Evolutionary patterns of cranial nerve efferent nuclei in vertebrates. Brain Behav. Evol. 2005, 66, 234–254.
- Pasqualetti, M.; Diaz, C.; Renaud, J.S.; Rijli, F.M.; Glover, J.C. Fate-mapping the mammalian hindbrain: Segmental origins of vestibular projection neurons assessed using rhombomere-specific Hoxa2 enhancer elements in the mouse embryo. J. Neurosci. 2007, 27, 9670–9681.
- Samad, O.A.; Geisen, M.J.; Caronia, G.; Varlet, I.; Zappavigna, V.; Ericson, J.; Goridis, C.; Rijli, F.M. Integration of anteroposterior and dorsoventral regulation of Phox2b transcription in cranial motoneuron progenitors by homeodomain proteins. Development 2004, 131, 4071–4083.
- Lumsden, A.; Keynes, R. Segmental patterns of neuronal development in the chick hindbrain. Nature 1989, 337, 424–428.
- Minoux, M.; Rijli, F.M. Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development 2010, 137, 2605–2621.
- Le Douarin, N. The Neural Crest; Cambridge University Press: Cambridge, UK, 1983.
- Le Douarin, N.; Kalcheim, C. The Neural Crest, 2nd ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1999; 445p.
- Keynes, R.; Lumsden, A. Segmentation and the origins of regional diversity in the vertebrate central nervous system. Neuron 1990, 4, 1–9.
- Guthrie, S.; Lumsden, A. Formation and regeneration of rhombomere boundaries in the developing chick hindbrain. Development 1991, 112, 221–229.
- Lumsden, A.; Krumlauf, R. Patterning the vertebrate neuraxis. Science 1996, 274, 1109–1115.
- Moens, C.B.; Prince, V.E. Constructing the hindbrain: Insights from the zebrafish. Dev. Dyn. 2002, 224.
- Moens, C.B.; Kimmel, C.B. Hindbrain patterning in the zebrafish embryo. Soc. Neurosci. Abstr. 1995, 21, 118.118.
- Moens, C.B.; Cordes, S.P.; Giorgianni, M.W.; Barsh, G.S.; Kimmel, C.B. Equivalence in the genetic control of hindbrain segmentation in fish and mouse. Development 1998, 125, 381–391.
- Waskiewicz, A.J.; Rikhof, H.A.; Moens, C.B. Eliminating zebrafish pbx proteins reveals a hindbrain ground state. Dev. Cell 2002, 3, 723–733.
- Kolm, P.; Apekin, V.; Sive, H. Xenopus hindbrain patterning requires retinoid signaling. Dev. Biol. 1997, 192, 1–16.
- Godsave, S.; Dekker, E.J.; Holling, T.; Pannese, M.; Boncinelli, E.; Durston, A. Expression patterns of Hoxb genes in the Xenopus embryo suggest roles in anteroposterior specification of the hindbrain and in dorsoventral patterning of the mesoderm. Dev. Biol. 1994, 166, 465–476.
- Wilkinson, D.G.; Bhatt, S.; Chavrier, P.; Bravo, R.; Charnay, P. Segment-specific expression of a zinc-finger gene in the developing nervous system of the mouse. Nature 1989, 337, 461–464.
- Xu, Q.; Mellitzer, G.; Robinson, V.; Wilkinson, D.G. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature 1999, 399, 267–271.
- Addison, M.; Xu, Q.; Cayuso, J.; Wilkinson, D.G. Cell Identity Switching Regulated by Retinoic Acid Signaling Maintains Homogeneous Segments in the Hindbrain. Dev. Cell 2018, 45, 606–620.e3.
- Morrison, A.; Chaudhuri, C.; Ariza-McNaughton, L.; Muchamore, I.; Kuroiwa, A.; Krumlauf, R. Comparative analysis of chicken Hoxb-4 regulation in transgenic mice. Mech. Dev. 1995, 53, 47–59.
- Gould, A.; Morrison, A.; Sproat, G.; White, R.A.; Krumlauf, R. Positive cross-regulation and enhancer sharing: Two mechanisms for specifying overlapping Hox expression patterns. Genes Dev. 1997, 11, 900–913.
- Manzanares, M.; Nardelli, J.; Gilardi-Hebenstreit, P.; Marshall, H.; Giudicelli, F.; Martinez-Pastor, M.T.; Krumlauf, R.; Charnay, P. Krox20 and kreisler co-operate in the transcriptional control of segmental expression of Hoxb3 in the developing hindbrain. EMBO J. 2002, 21, 365–376.
- Manzanares, M.; Bel-Vialer, S.; Ariza-McNaughton, L.; Ferretti, E.; Marshall, H.; Maconochie, M.K.; Blasi, F.; Krumlauf, R. Independent regulation of initiation and maintenance phases of Hoxa3 expression in the vertebrate hindbrain involves auto and cross-regulatory mechanisms. Development 2001, 128, 3595–3607.
- Nonchev, S.; Maconochie, M.; Vesque, C.; Aparicio, S.; Ariza-McNaughton, L.; Manzanares, M.; Maruthainar, K.; Kuroiwa, A.; Brenner, S.; Charnay, P.; et al. The conserved role of Krox-20 in directing Hox gene expression during vertebrate hindbrain segmentation. Proc. Natl. Acad. Sci. USA 1996, 93, 9339–9345.
- Nonchev, S.; Vesque, C.; Maconochie, M.; Seitanidou, T.; Ariza-McNaughton, L.; Frain, M.; Marshall, H.; Sham, M.H.; Krumlauf, R.; Charnay, P. Segmental expression of Hoxa-2 in the hindbrain is directly regulated by Krox-20. Development 1996, 122, 543–554.
- Vesque, C.; Maconochie, M.; Nonchev, S.; Ariza-McNaughton, L.; Kuroiwa, A.; Charnay, P.; Krumlauf, R. Hoxb-2 transcriptional activation in rhombomeres 3 and 5 requires an evolutionarily conserved cis-acting element in addition to the Krox-20 binding site. EMBO J. 1996, 15, 5383–5396.
- Sham, M.-H.; Hunt, P.; Nonchev, S.; Papalopulu, N.; Graham, A.; Boncinelli, E.; Krumlauf, R. Analysis of the murine Hox-2.7 gene: Conserved alternative transcripts with differential distributions in the nervous system and the potential for shared regulatory regions. EMBO J. 1992, 11, 1825–1836.
- Morrison, A.; Moroni, M.C.; Ariza-McNaughton, L.; Krumlauf, R.; Mavilio, F. In vitro and transgenic analysis of a human HOXD4 retinoid-responsive enhancer. Development 1996, 122, 1895–1907.
- Popperl, H.; Bienz, M.; Studer, M.; Chan, S.K.; Aparicio, S.; Brenner, S.; Mann, R.S.; Krumlauf, R. Segmental expression of Hoxb-1 is controlled by a highly conserved autoregulatory loop dependent upon exd/pbx. Cell 1995, 81, 1031–1042.
- Marshall, H.; Studer, M.; Popperl, H.; Aparicio, S.; Kuroiwa, A.; Brenner, S.; Krumlauf, R. A conserved retinoic acid response element required for early expression of the homeobox gene Hoxb-1. Nature 1994, 370, 567–571.
- Parker, H.J.; Bronner, M.E.; Krumlauf, R. A Hox regulatory network of hindbrain segmentation is conserved to the base of vertebrates. Nature 2014, 514, 490–493.
- McNulty, C.L.; Peres, J.N.; Bardine, N.; van den Akker, W.M.; Durston, A.J. Knockdown of the complete Hox paralogous group 1 leads to dramatic hindbrain and neural crest defects. Development 2005, 132, 2861–2871.
- Tvrdik, P.; Capecchi, M.R. Reversal of hox1 gene subfunctionalization in the mouse. Dev. Cell 2006, 11, 239–250.
- Rijli, F.; Gavalas, A.; Chambon, P. Segmentation and specification in the branchial region of the head: The role of Hox selector genes. Int. J. Dev. Biol. 1998, 42, 393–401.
- Dupe, V.; Davenne, M.; Brocard, J.; Dolle, P.; Mark, M.; Dierich, A.; Chambon, P.; Rijli, F.M. In vivo functional analysis of the Hoxa-1 3’ retinoic acid response element (3′RARE). Development 1997, 124, 399–410.
- Rijli, F.M.; Mark, M.; Lakkaraju, S.; Dierich, A.; Dollé, P.; Chambon, P. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell 1993, 75, 1333–1349.
- Parker, H.J.; Pushel, I.; Krumlauf, R. Coupling the roles of Hox genes to regulatory networks patterning cranial neural crest. Dev. Biol. 2018, 444 (Suppl. 1), S67–S78.
- Baltzinger, M.; Ori, M.; Pasqualetti, M.; Nardi, I.; Rijli, F.M. Hoxa2 knockdown in Xenopus results in hyoid to mandibular homeosis. Dev. Dyn. 2005, 234, 858–867.
- Santagati, F.; Minoux, M.; Ren, S.Y.; Rijli, F.M. Temporal requirement of Hoxa2 in cranial neural crest skeletal morphogenesis. Development 2005, 132, 4927–4936.
- Kulesa, P.M.; Fraser, S.E. In ovo time-lapse analysis of chick hindbrain neural crest cell migration shows cell interactions during migration to the branchial arches. Development 2000, 127, 1161–1172.
- Trainor, P.A.; Sobieszczuk, D.; Wilkinson, D.; Krumlauf, R. Signalling between the hindbrain and paraxial tissues dictates neural crest migration pathways. Development 2002, 129, 433–442.
- Graham, A.; Heyman, I.; Lumsden, A. Even-numbered rhombomeres control the apoptotic elimination of neural crest cells from odd-numbered rhombomeres in the chick hindbrain. Development 1993, 119, 233–245.
- Sechrist, J.; Serbedzija, G.N.; Scherson, T.; Fraser, S.E.; Bronner-Fraser, M. Segmental migration of the hindbrain neural crest does not arise from its segmental generation. Development 1993, 118, 691–703.
- Maconochie, M.; Nonchev, S.; Morrison, A.; Krumlauf, R. Paralogous Hox genes: Function and regulation. Annu. Rev. Genet. 1996, 30, 529–556.
- Schilling, T.F.; Nie, Q.; Lander, A.D. Dynamics and precision in retinoic acid morphogen gradients. Curr. Opin. Genet. Dev. 2012, 22, 562–569.
- Parker, H.J.; Bronner, M.E.; Krumlauf, R. The vertebrate Hox gene regulatory network for hindbrain segmentation: Evolution and diversification: Coupling of a Hox gene regulatory network to hindbrain segmentation is an ancient trait originating at the base of vertebrates. BioEssays 2016, 38, 526–538.
- Sauka-Spengler, T.; Meulemans, D.; Jones, M.; Bronner-Fraser, M. Ancient evolutionary origin of the neural crest gene regulatory network. Dev. Cell 2007, 13, 405–420.
- Parker, H.J.; De Kumar, B.; Green, S.A.; Prummel, K.D.; Hess, C.; Kaufman, C.K.; Mosimann, C.; Wiedemann, L.M.; Bronner, M.E.; Krumlauf, R. A Hox-TALE regulatory circuit for neural crest patterning is conserved across vertebrates. Nat. Commun. 2019, 10, 1189.
- Buckingham, M.; Meilhac, S.; Zaffran, S. Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 2005, 6, 826–835.
- Vincent, S.D.; Buckingham, M.E. How to make a heart: The origin and regulation of cardiac progenitor cells. Curr. Top. Dev. Biol. 2010, 90, 1–41.
- Uehara, M.; Yashiro, K.; Takaoka, K.; Yamamoto, M.; Hamada, H. Removal of maternal retinoic acid by embryonic CYP26 is required for correct Nodal expression during early embryonic patterning. Genes Dev. 2009, 23, 1689–1698.
- Kudoh, T.; Wilson, S.W.; Dawid, I.B. Distinct roles for Fgf, Wnt and retinoic acid in posteriorizing the neural ectoderm. Development 2002, 129, 4335–4346.
- Kam, R.K.; Deng, Y.; Chen, Y.; Zhao, H. Retinoic acid synthesis and functions in early embryonic development. Cell Biosci. 2012, 2, 11.
- Rhinn, M.; Dolle, P. Retinoic acid signalling during development. Development 2012, 139, 843–858.
- Niederreither, K.; Dolle, P. Retinoic acid in development: Towards an integrated view. Nat. Rev. Genet. 2008, 9, 541–553.
- Evans, R.M.; Mangelsdorf, D.J. Nuclear Receptors, RXR, and the Big Bang. Cell 2014, 157, 255–266.
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schutz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839.
- Xu, Q.; Jitkaew, S.; Choksi, S.; Kadigamuwa, C.; Qu, J.; Choe, M.; Jang, J.; Liu, C.; Liu, Z.G. The cytoplasmic nuclear receptor RARgamma controls RIP1 initiated cell death when cIAP activity is inhibited. Nat. Commun. 2017, 8, 425.
More
Information
Subjects:
Ecology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
844
Revisions:
2 times
(View History)
Update Date:
18 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




