Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Robb Krumlauf and Version 2 by Lily Guo.
In metazoans, Hox genes are key drivers of morphogenesis. In chordates, they play important roles in patterning the antero-posterior (A-P) axis. A crucial aspect of their role in axial patterning is their collinear expression, a process thought to be linked to their response to major signaling pathways such as retinoic acid (RA) signaling. The amplification of Hox genes following major events of genome evolution can contribute to morphological diversity. In vertebrates, RA acts as a key regulator of the gene regulatory network (GRN) underlying hindbrain segmentation, which includes Hox genes.
- hindbrain
- segmentation
1. Introduction
In metazoans the Hox family of transcription factors (TFs) play important roles in patterning antero-posterior (A-P) identity along the body axis [1][2][3][4][5][6][7][8][9][10][1,2,3,4,5,6,7,8,9,10]. In most organisms, Hox genes are present in the genome in tightly linked chromosomal clusters, and display highly conserved features in their organization, expression, and function [10][11][12][13][14][15][16][17][18][19][10,11,12,13,14,15,16,17,18,19]. An important property of the clustered Hox genes is collinearity (Figure 1a), which refers to their highly ordered spatial and temporal patterns of expression along the A-P axis during embryogenesis [13][20][21][22][13,20,21,22]. In any given Hox cluster, the gene located on one end of the cluster, usually Hox1, is expressed in a domain that arises early, with an anterior boundary that maps in the head region, and each successive adjacent gene in the cluster is progressively expressed later and more posteriorly (e.g., Hox2 to 15) [2][10][11][12][13][18][19][23][24][25][26][27][28][29][2,10,11,12,13,18,19,23,24,25,26,27,28,29]. This spatial and temporal program of gene expression sets up precisely ordered and nested domains of the Hox TFs along the A-P axis which form a molecular code, referred to as the ‘Hox code’. This combinatorial Hox code is used to specify and pattern different regional characteristics of tissues and structures along the A-P axis [1][2][3][4][24][30][31][32][1,2,3,4,24,30,31,32].
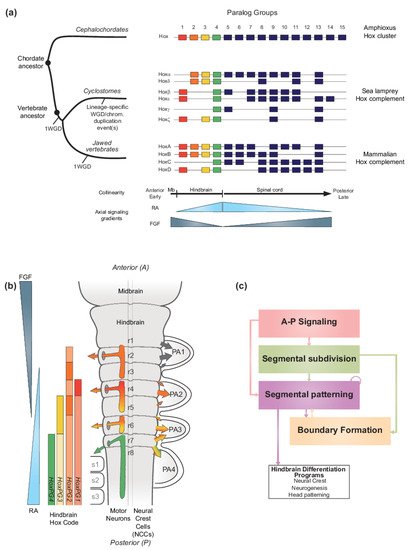
FigureFigure 1. 1.Hox genes and vertebrate hindbrain segmentation. Hox genes and vertebrate hindbrain segmentation. (a) Schematic representation of the evolution of Hox clusters in different chordate models-amphioxus, sea lamprey and mouse, illustrating the different ways gene families can evolve following major genome rearrangements. Amphioxus displays one Hox cluster, suggesting that the chordate ancestor likely possessed one Hox cluster. In vertebrates, multiple whole genome duplication (WGD) and/or chromosomal scale duplication events, led to the amplification of the ancestral Hox cluster which is thought to have been important for generating morphological diversity. Resulting Hox complements are depicted, and paralog groups (PG) are numbered 1 to 15. HoxPG1–PG4 are color-coded to reflect their role in axial patterning of the developing brain region, including in the vertebrate hindbrain. In the early diverged jawless vertebrate group (cyclostomes), the sea lamprey Hox complement is composed of 6 clusters denoted Hoxα to ξ and Hox clusters are displayed to reflect their putative evolutionary history [33]. In contrast, in jawed vertebrates, the mammalian Hox complement is composed of 4 Hox clusters and denoted HoxA to D. The colinear property of Hox gene expression is thought to be directly linked to their response to major signaling gradients acting as morphogens such as Retinoic Acid (RA) and Fibroblast Growth Factor (FGF). In vertebrates, the colinear expression of HoxPG1-PG4 is important for the segmentation of the hindbrain. (b) Schematic representation of the segmented vertebrate hindbrain and important structures emanating from individual segments (rhombomeres (r)). In the hindbrain, Hox PG1–PG4 establish a ‘Hindbrain Hox code’ in response to RA and FGF, which strongly influences hindbrain segmentation. The Hindbrain Hox code is represented following the HoxPG1–PG4 color code, with darker shades representing higher levels of expression in specific rhombomeres. The influence of the Hox code in segmentally derived motor neurons and neural crest cells (NCCs) migrating into the pharyngeal arches (PA) is represented by colored arrows. Streams of NCCs migrating into PA1 are not influenced by the Hox code (black arrows). Panel modified from [34]. (c) Diagram representing the vertebrate hindbrain Gene Regulatory Network (GRN). The Hox code and signaling gradients constitute important aspects of a broader GRN underlying hindbrain segmentation, composed of different color-coded modules. Colored arrows indicate the regulatory relationships between different modules.
In vertebrates, the Hox code is tightly coupled to segmentation of the hindbrain (Figure 1b) [35][36][37][38][39][40][35,36,37,38,39,40] and the axial skeleton [4][32][41][4,32,41]. Functional studies in different species have demonstrated that, during segmentation, the Hox code sets up a molecular representation of the morphological ground plan along the A-P axis in the central nervous system (CNS) and mesoderm that regulates patterning, differentiation and wiring of hindbrain neural programs and specification of different vertebral identities [4][32][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][4,32,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63]. Pan-vertebrate whole genome duplication (WGD) in combination with putative lineage specific WGD and/or chromosomal duplication event(s) led to the amplification and divergence of Hox genes in the evolution of vertebrates from a single ancestral cluster at the origin of chordates (Figure 1a) [10][64][65][66][67][68][69][70][71][72][73][10,64,65,66,67,68,69,70,71,72,73]. As a result of at least one WGD (as discussed later), all vertebrates evolved to have four or more Hox clusters organized into 14 paralog groups. In the jawed vertebrate group, mammals have four Hox clusters while teleost fishes have 7–8 Hox clusters [74]. In the early diverged jawless vertebrate group, the cyclostomes, lampreys and hagfish have six Hox clusters (Figure 1a), indicating that additional duplication events have shaped cyclostome genomes [72][75][76][72,75,76]. In light of their ancient and fundamental roles in regulating morphogenesis and A-P patterning, the amplification and divergence of Hox complements in vertebrates is thought to be a driver in the emergence of new traits and evolutionary novelties by being integrated in gene regulatory networks underlying morphological diversity [2][62][77][78][2,62,77,78]
Diverse molecular and cellular mechanisms are thought to contribute to the regulation and generation of collinear domains of Hox expression [20][21][79][80][81][82][83][84][85][86][20,21,79,80,81,82,83,84,85,86]. Experimental studies in a wide range of vertebrates and invertebrates have revealed that collinear Hox expression arises in part through the ability of Hox clusters to integrate information from A-P signaling centers in response to cues from major signaling pathways, such as retinoic acid (RA), fibroblast growth factor (FGF), and Wnts [21][87][88][89][90][91][92][93][21,87,88,89,90,91,92,93]. For example, in vertebrate model systems, opposing gradients of RA and FGFs have been shown to regulate nested domains of Hox expression in the CNS and in mesodermal domains that control specification of vertebral identities and axial elongation (Figure 1a,b) [91][94][95][96][97][98][99][91,94,95,96,97,98,99]. In the hindbrain, RA signaling plays a key role in triggering the process of segmentation and establishes segmental patterning of Hox expression [43][91][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118][119][120][121][122][123][124][43,91,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124]. In addition, in cultured cells differentiated into neural fates, Hox genes display temporal collinearity in response to treatment with RA [125][126][127][128][129][125,126,127,128,129]. This wealth of experimental data reveals a high degree of functional coupling between Hox genes and signaling pathways and suggests that this is a fundamental feature of their clustered organization which underlies the collinearity of their expression patterns.
Beyond Hox gene, evolutionary analyses have revealed that the expression domains of many genes encoding important developmental TFs and components of key signaling pathways (FGF, Hh and Wnt) are similarly aligned along the A-P axis of hemichordates and chordates [23][31][77][130][131][23,31,77,130,131]. This suggests that, despite very different morphologies between phyla, a deeply conserved A-P patterning system, integrating axial signaling pathways and Hox genes, may have evolved long ago in deuterostome evolution. The diversity in morphological outcomes from this ancient axial patterning system are likely to arise through differences in the downstream targets of the conserved TFs and in signals that direct the terminal differentiation programs for organogenesis and morphogenesis. Hence, the ability of vertebrate Hox clusters to coordinately respond to RA signaling in many tissues may be related to their participation in a broader and ancient regulatory mechanism that underlies the patterning of regional diversity along the A-P axis in animal development.
Understanding how Hox genes and components of the ancient A-P patterning system are regulated by signaling pathways is important for investigating the evolution and emergence of A-P patterning mechanisms in the vertebrate lineage. Indeed, vertebrates may have co-opted this ancient patterning system and coupled it to novel programs of patterning and differentiation, such as hindbrain segmentation, which may have contributed to morphological diversity. The vertebrate hindbrain is an excellent model to investigate how programs of A-P patterning are coupled to signaling gradients and to explore how changes in this coupling during evolution may be associated with the emergence of morphological diversity and complexity. In vertebrates, division of the brain into different compartments during development shows considerable variability between species, but the hindbrain is a complex coordination center that displays a remarkably high degree of conservation in all vertebrates. The hindbrain contains a sophisticated network of neural circuits that play essential roles in controlling many physiological processes and behaviors [42][43][47][49][55][56][57][58][132][133][134][135][136][137][42,43,47,49,55,56,57,58,132,133,134,135,136,137]. It also plays a central role in organization of the head and craniofacial tissues through the generation of cranial neural crest cells [138][139][140][138,139,140].
During embryogenesis, the basic ground plan of the hindbrain is established through a process of segmentation, which organizes the region into a series of seven transient segments named rhombomeres (r) (Figure 1b) [37][38][43][137][141][142][143][144][145][146][147][148][149][150][151][152][37,38,43,137,141,142,143,144,145,146,147,148,149,150,151,152]. Genes in the Hox1–4 paralog groups (Hox PG1–PG4) are coupled to the process of segmentation and display segmentally restricted domains of expression, resulting in a ‘hindbrain Hox code’ [36][37][115][117][149][153][154][155][156][157][158][159][160][161][162][163][164][165][166][36,37,115,117,149,153,154,155,156,157,158,159,160,161,162,163,164,165,166]. This code ultimately confers each rhombomere with a unique molecular identity that regulates programs of neurogenesis and elaboration of the neural circuitry associated with its distinct functions in the hindbrain [42][43][47][49][58][132][133][134][135][136][137][143][167][168][42,43,47,49,58,132,133,134,135,136,137,143,167,168]. Disruption of this code results in dramatic perturbations of hindbrain and head development [51][147][169][170][171][172][51,147,169,170,171,172]. The formation of cranial neural crest cells, whose differentiated derivatives generate most of the bone and connective tissues of the head, is also coupled to hindbrain segmentation [138]. The cranial neural crest cells delaminate and migrate from the mid/hindbrain region in an organized manner [173][174][175][176][173,174,175,176], and then differentiate to form peripheral target tissues and cranial ganglia that are in register with the segmentally organized branchiomotor and reticulospinal neurons (Figure 1b). Hence, hindbrain segmentation also makes an important contribution to global head development and craniofacial patterning.
Analyses of patterns of gene expression, phenotypes arising from mutations and perturbation of expression and characterization of cis-regulatory elements in many different vertebrate species, particularly zebrafish, Xenopus, chicken, and mouse embryos, have revealed that the regulatory basis for establishing the Hox code is embedded in a conserved gene regulatory network (GRN) underlying hindbrain segmentation [34][38][42][43][100][101][103][143][144][177][178][34,38,42,43,100,101,103,143,144,177,178]. For example, a detailed list of cis-regulatory modules, activities, regulatory inputs, and species of origin used for constructing this GRN can be found in Table 1 of reference [34]. This GRN may be represented or visualized as a dynamic series of progressive steps or modules associated with the respective cell and developmental processes they regulate (Figure 1c) [43][100][43,100]. This GRN also provides a framework for understanding how signaling gradients and TFs, such as RA and Hox, are integrated into regulatory circuits that form and pattern hindbrain segments with distinct A-P identities. The FGF, Wnt, and RA signaling pathways govern initial steps of the GRN for hindbrain segmentation [93] and signaling cues from these pathways act as morphogens to dictate the molecular identity and organization of cells along the A-P axis. The RA morphogen is part of a well characterized signaling pathway which is of particular importance in precisely regulating the expression of key TFs, including Hox genes, in multiple modules in the GRN.
From an evolutionary perspective, hindbrain segmentation is a trait uniquely found in the vertebrate group and is remarkably conserved in all characterized vertebrate lineages [38][43][38,43]. Hindbrain segmentation appears to be an important vertebrate innovation that, in concert with the ability to form neural crest cells, is wired into conserved GRNs for developmental programs governing head development [34][100][164][179][180][34,100,164,179,180]. Recent studies in jawless vertebrates, such as lamprey, have revealed that many core components and TFs in the hindbrain GRN are deeply conserved to the base of the vertebrate tree [164][179][181][164,179,181], however, much less is known about the level of conservation of the roles of signaling pathways in the GRN of this vertebrate group. Because of their unique position as part of an early diverged vertebrate group, jawless vertebrate models provide an important opportunity to examine whether RA played an analogous role in different aspects of the hindbrain GRN early in vertebrate evolution and how this role might have evolved.
2. RA Signaling and Vertebrate Hindbrain Segmentation
2.1. RA Signaling and Its Roles in Development
RA signaling plays important roles in many fundamental biological processes. Cues from this pathway are involved in regulation of cell growth and proliferation, differentiation, and the control of homeostasis in multiple tissues. In development, RA can act as a morphogen, i.e., a signaling molecule that forms a concentration gradient over space and time which activates target genes in a concentration-dependent manner. In early vertebrate embryogenesis, RA is involved in the regulation of heart development [182][183][182,183], body axis formation [94][113][184][94,113,184] and the patterning and elongation of the A-P axis, through interactions with other signaling gradients (FGF and Wnt) [94][97][185][94,97,185]. In addition, RA signaling provides regulatory inputs into neural differentiation programs [113][186][113,186], pancreas specification and eye, kidney, and lung development [186][187][188][186,187,188]. Furthermore, RA signaling has a well-established role in A-P patterning of the hindbrain, where it contributes to the dynamic regulation of the process of segmentation, which lays down a basic ground plan for the elaboration of this key coordination center in the brain [43][100][103][144][178][43,100,103,144,178].
The RA signaling pathway can be broken down into three general steps: (1) RA metabolism to generate active ligands, (2) RA signal transduction to modulate gene expression, and (3) RA degradation to control levels of active ligand (Figure 2a). Components of the RA signaling pathway involve a Vitamin A precursor, binding proteins that mediate its extra- and intracellular transport, metabolic enzymes that convert it to an active ligand (e.g., all-trans RA and 9-cis RA) and enzymes that control the degradation of RA (Figure 2a). Signal transduction is mediated by the interaction of RA with members of the nuclear hormone receptor family of proteins (RAR and RXR) which bind directly to DNA regulatory elements in the genome and modulate patterns of gene expression [189][190][189,190]. The synthesizing and degrading enzymes, transport proteins, receptors and DNA regulatory elements together constitute the RA machinery and provide multiple opportunities for evolving and regulating the RA signaling pathway in various biological processes [187][188][187,188]. In this section, we summarize what is known about components of the RA signaling pathway and relate it to hindbrain segmentation, Hox genes, and the evolution of A-P patterning in vertebrates. The primary focus of this review is on the canonical pathway of RA signaling. However, there is emerging evidence concerning the non-canonical functions of certain components of the pathway, such as the cytoplasmic function of RARγ in cell death [191].
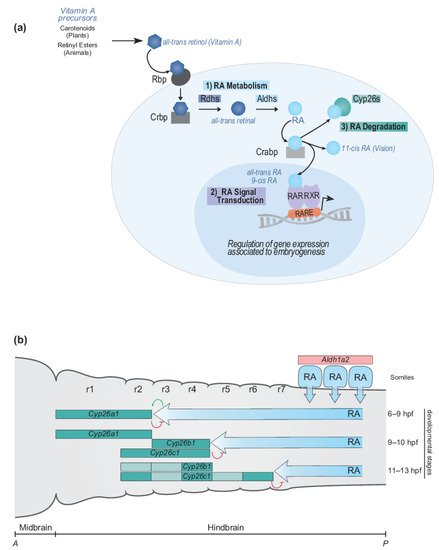
FigureFigure 2. 2.RA signaling pathway and RA morphogen gradient during hindbrain development. RA signaling pathway and RA morphogen gradient during hindbrain development. (a) Simplified schematic representation of major steps of the RA signaling pathway and main components of the RA machinery. The RA signaling pathway converts Vitamin A to RA following three important steps: (1) RA metabolism, (2) RA signal transduction and (3) RA degradation. The RA machinery includes main actors of the signaling pathway: RA synthesizing and degrading enzymes (Rdhs; Aldhs; Cyp26s), transport proteins (Rbp; Crbp; Crabp), nuclear hormone receptors (RAR-RXRs), and Retinoic Acid Response Elements (RAREs). (b) Schematic depiction of the dynamic regulation of endogenous levels of RA during hindbrain development. The RA morphogen gradient is set up by synthesizing and degrading enzymes of the RA machinery in the context of the vertebrate hindbrain. Aldh1a2 is expressed in the somites where Aldh1a2 localizes and synthesizes RA. The spatially and temporally dynamic segmental expression of Cyp26a1, Cyp26b1, and Cyp26c1 induces shifting domains of Cyp26 degrading activity, which act as anterior sinks of RA activity, therefore generating a precise and gradual set up of the RA morphogen gradient. The developmental time points correspond to zebrafish development. A, anterior; P, posterior; r, rhombomere.
