
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Karen Bohmwald | + 2016 word(s) | 2016 | 2022-01-12 04:56:09 | | | |
| 2 | Beatrix Zheng | Meta information modification | 2016 | 2022-01-18 02:50:41 | | |
Video Upload Options
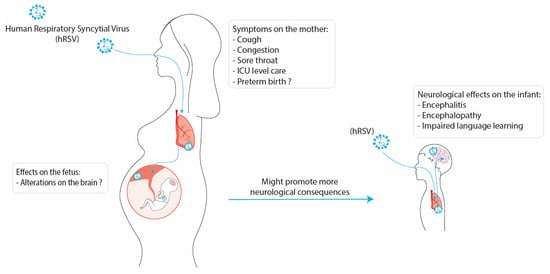
Respiratory infections are among the major public health burdens, especially during winter. Along these lines, the human respiratory syncytial virus (hRSV) is the principal viral agent causing acute lower respiratory tract infections leading to hospitalization. The pulmonary manifestations due to hRSV infection are bronchiolitis and pneumonia, where the population most affected are infants and the elderly. However, recent evidence suggests that hRSV infection can impact the mother and fetus during pregnancy. Studies have indicated that hRSV can infect different cell types from the placenta and even cross the placenta barrier and infect the fetus. In addition, it is known that infections during the gestational period can lead to severe consequences for the development of the fetus due not only to a direct viral infection but also because of maternal immune activation (MIA). Furthermore, it has been described that the development of the central nervous system (CNS) of the fetus can be affected by the inflammatory environment of the uterus caused by viral infections. Increasing evidence supports the notion that hRSV could invade the CNS and infect nervous cells, such as microglia, neurons, and astrocytes, promoting neuroinflammation. Moreover, it has been described that the hRSV infection can provoke neurological manifestations, including cognitive impairment and behavioral alterations.
1. Introduction
2. Neurological Consequences due to hRSV Infection
3. Conclusions
References
- Barr, R.; Green, C.A.; Sande, C.J.; Drysdale, S.B. Respiratory syncytial virus: Diagnosis, prevention and management. Ther. Adv. Infect. Dis. 2019, 6, 204993611986579.
- Caballero, M.T.; Polack, F.P. Respiratory syncytial virus is an “opportunistic” killer. Pediatr. Pulmonol. 2018, 53, 664–667.
- Mammas, I.N.; Drysdale, S.B.; Rath, B.; Theodoridou, M.; Papaioannou, G.; Papatheodoropoulou, A.; Koutsounaki, E.; Koutsaftiki, C.; Kozanidou, E.; Achtsidis, V.; et al. Update on current views and advances on RSV infection (Review). Int. J. Mol. Med. 2020, 46, 509–520.
- Amarasinghe, G.K.; Ayllón, M.A.; Bào, Y.; Basler, C.F.; Bavari, S.; Blasdell, K.R.; Briese, T.; Brown, P.A.; Bukreyev, A.; Balkema-Buschmann, A.; et al. Taxonomy of the order Mononegavirales: Update 2019. Arch. Virol. 2019, 164, 1967–1980.
- Bohmwald, K.; Espinoza, J.A.; Rey-Jurado, E.; Gómez, R.S.; González, P.A.; Bueno, S.M.; Riedel, C.A.; Kalergis, A.M. Human Respiratory Syncytial Virus: Infection and Pathology. Semin. Respir. Crit. Care Med. 2016, 37, 522–537.
- Andeweg, S.P.; Schepp, R.M.; van de Kassteele, J.; Mollema, L.; Berbers, G.A.M.; van Boven, M. Population-based serology reveals risk factors for RSV infection in children younger than 5 years. Sci. Rep. 2021, 11, 8953.
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958.
- Kazakova, A.; Kakkola, L.; Päkkilä, H.; Teros-Jaakkola, T.; Soukka, T.; Peltola, V.; Waris, M.; Julkunen, I. Serological Array-in-Well Multiplex Assay Reveals a High Rate of Respiratory Virus Infections and Reinfections in Young Children. mSphere 2019, 4, e00447-19.
- Hervé, P.L.; Deloizy, C.; Descamps, D.; Rameix-Welti, M.A.; Fix, J.; McLellan, J.S.; Eléouët, J.F.; Riffault, S. RSV N-nanorings fused to palivizumab-targeted neutralizing epitope as a nanoparticle RSV vaccine. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 411–420.
- Opek, M.W.; Yeshayahu, Y.; Glatman-Freedman, A.; Kaufman, Z.; Sorek, N.; Brosh-Nissimov, T. Delayed respiratory syncytial virus epidemic in children after relaxation of COVID-19 physical distancing measures, Ashdod, Israel, 2021. Eurosurveillance 2021, 26, 2100706.
- Agha, R.; Avner, J.R. Delayed Seasonal RSV Surge Observed During the COVID-19 Pandemic. Pediatrics 2021, 148, e2021052089.
- Foley, D.A.; Yeoh, D.K.; Minney-Smith, C.A.; Martin, A.C.; Mace, A.O.; Sikazwe, C.T.; Le, H.; Levy, A.; Moore, H.C.; Blyth, C.C. The Interseasonal Resurgence of Respiratory Syncytial Virus in Australian Children Following the Reduction of Coronavirus Disease 2019–Related Public Health Measures. Clin. Infect. Dis. 2021, 73, e2829–e2830.
- Ujiie, M.; Tsuzuki, S.; Nakamoto, T.; Iwamoto, N. Resurgence of Respiratory Syncytial Virus Infections during COVID-19 Pandemic, Tokyo, Japan. Emerg. Infect. Dis. 2021, 27, 2969–2970.
- Kalergis, A.M.; Soto, J.A.; Gálvez, N.M.S.; Andrade, C.A.; Fernandez, A.; Bohmwald, K.; Bueno, S.M. Pharmacological management of human respiratory syncytial virus infection. Expert Opin. Pharmacother. 2020, 21, 2293–2303.
- Mac, S.; Sumner, A.; Duchesne-Belanger, S.; Stirling, R.; Tunis, M.; Sander, B. Cost-effectiveness of Palivizumab for Respiratory Syncytial Virus: A systematic review. Pediatrics 2019, 143, e20184064.
- Andrade, C.A.; Pacheco, G.A.; Gálvez, N.M.S.; Soto, J.A.; Bueno, S.M.; Kalergis, A.M. Innate immune components that regulate the pathogenesis and resolution of hRSV and hMPV infections. Viruses 2020, 12, 637.
- Bohmwald, K.; Soto, J.A.J.A.; Andrade-Parra, C.; Fernández-Fierro, A.; Espinoza, J.A.J.A.; Ríos, M.; Eugenin, E.A.E.A.E.A.; González, P.A.P.A.; Opazo, M.C.M.C.; Riedel, C.A.; et al. Lung pathology due to hRSV infection impairs blood–brain barrier permeability enabling astrocyte infection and a long-lasting inflammation in the CNS. Brain. Behav. Immun. 2021, 91, 159–171.
- Velázquez-Cervantes, M.A.; Martínez-Castillo, M.; González-García, L.D.; Vargas-Pavía, T.A.; Martínez-Salazar, M.G.; Mancilla-Herrera, I.; León-Reyes, G.; García-Cordero, J.; Helguera-Repetto, A.C.; León-Juárez, M. The BeWo cell line derived from a human placental choriocarcinoma is permissive for respiratory syncytial virus infection. Virus Genes 2019, 55, 406–410.
- Bohmwald, K.; Gálvez, N.M.S.; Ríos, M.; Kalergis, A.M. Neurologic Alterations Due to Respiratory Virus Infections. Front. Cell. Neurosci. 2018, 12, 386.
- Park, A.; Suh, S.I.; Son, G.R.; Lee, Y.H.; Seo, H.S.; Eun, B.L.; Lee, N.J.; Seol, H.Y. Respiratory syncytial virus-related encephalitis: Magnetic resonance imaging findings with diffusion-weighted study. Neuroradiology 2014, 56, 163–168.
- Xu, L.; Gao, H.; Zeng, J.; Liu, J.; Lu, C.; Guan, X.; Qian, S.; Xie, Z. A fatal case associated with respiratory syncytial virus infection in a young child. BMC Infect. Dis. 2018, 18, 217.
- Regan, A.K.; Klein, N.P.; Langley, G.; Drews, S.J.; Buchan, S.; Ball, S.; Kwong, J.C.; Naleway, A.; Thompson, M.; Wyant, B.E.; et al. Respiratory Syncytial Virus Hospitalization During Pregnancy in 4 High-income Countries, 2010–2016. Clin. Infect. Dis. 2018, 67, 1915–1918.
- Wheeler, S.M.; Dotters-Katz, S.; Heine, R.P.; Grotegut, C.A.; Swamy, G.K. Maternal Effects of Respiratory Syncytial Virus Infection during Pregnancy. Emerg. Infect. Dis. 2015, 21, 1951.
- Piedimonte, G.; Walton, C.; Samsell, L. Vertical Transmission of Respiratory Syncytial Virus Modulates Pre- and Postnatal Innervation and Reactivity of Rat Airways. PLoS ONE 2013, 8, e61309.
- Zeafley, J. Neurological, Electroencephalographic, and Virological Findings in Febrile Children Ages at Admission, and Sex Incidence Age at Time of Group A Group B Group C Group D Admission (28 cases) (25 cases) (18 cases) (7 cases). Arch. Dis. Child. 1970, 45, 611.
- Bohmwald, K.; Espinoza, J.A.; González, P.A.; Bueno, S.M.; Riedel, C.A.; Kalergis, A.M. Central nervous system alterations caused by infection with the human respiratory syncytial virus. Rev. Med. Virol. 2014.
- Tison-Chambellan, C.; Cheuret, E.; Cances, C.; Karsenty, C.; Le Camus, C.; Sevely, A.; Chaix, Y. Rhombencéphalite liée au virus respiratoire syncytial chez un garçon de 7 ans. Arch. Pediatr. 2013, 20, 657–660.
- Moriyama, K.; Takahashi, Y.; Shiihara, T. Another case of respiratory syncytial virus-related limbic encephalitis. Neuroradiology 2014, 56, 435–436.
- Millichap, J.J.; Wainwright, M.S. Neurological complications of respiratory syncytial virus infection: Case series and review of literature. J. Child Neurol. 2009, 24, 1499–1503.
- Kawashima, H.; Ioi, H.; Ushio, M.; Yamanaka, G.; Matsumoto, S.; Nakayama, T. Cerebrospinal fluid analysis in children with seizures from respiratory syncytial virus infection. Scand. J. Infect. Dis. 2009, 41, 228–231.
- Otake, Y.; Yamagata, T.; Morimoto, Y.; Imi, M.; Mori, M.; Aihara, T.; Ichiyama, T.; Momoi, M.Y. Elevated CSF IL-6 in a patient with respiratory syncytial virus encephalopathy. Brain Dev. 2007, 29, 117–120.
- Saravanos, G.L.; King, C.L.; Deng, L.; Dinsmore, N.; Ramos, I.; Takashima, M.; Crawford, N.; Clark, J.E.; Dale, R.C.; Jones, C.A.; et al. Respiratory Syncytial Virus–Associated Neurologic Complications in Children: A Systematic Review and Aggregated Case Series. J. Pediatr. 2021, 239, 39–49.e9.
- Picone, S.; Mondì, V.; Di Palma, F.; Martini, L.; Paolillo, P. Neonatal Encephalopathy and SIADH during RSV Infection. Am. J. Perinatol. 2019, 36, S106–S109.
- Van den Pol, A.N. van den Viral infection leading to brain dysfunction: More prevalent than appreciated? Neuron 2009, 64, 17.
- Peña, M.; Jara, C.; Flores, J.C.; Hoyos-Bachiloglu, R.; Iturriaga, C.; Medina, M.; Carcey, J.; Espinoza, J.; Bohmwald, K.; Kalergis, A.M.; et al. Severe respiratory disease caused by human respiratory syncytial virus impairs language learning during early infancy. Sci. Rep. 2020, 10, 22356.
- Bird, C.M.; Burgess, N. The hippocampus and memory: Insights from spatial processing. Nat. Rev. Neurosci. 2008, 9, 182–194.
- Miyamoto, K.; Fujisawa, M.; Tsuboi, T.; Hirao, J.I.; Sugita, K.; Arisaka, O.; Hozumi, H.; Kuwashima, S.; Tsuboi, T. Systemic inflammatory response syndrome and prolonged hypoperfusion lesions in an infant with respiratory syncytial virus encephalopathy. J. Infect. Chemother. 2013, 19, 978–982.
- Bogaerts, L.; Szmalec, A.; Hachmann, W.M.; Page, M.P.A.; Duyck, W. Linking memory and language: Evidence for a serial-order learning impairment in dyslexia. Res. Dev. Disabil. 2015, 43–44, 106–122.
- Espinoza, J.A.; Bohmwald, K.; Cespedes, P.F.; Gomez, R.S.; Riquelme, S.A.; Cortes, C.M.; Valenzuela, J.A.; Sandoval, R.A.; Pancetti, F.C.; Bueno, S.M.; et al. Impaired learning resulting from Respiratory Syncytial Virus infection. Proc. Natl. Acad. Sci. USA 2013, 11, 9112–9117.
- Kumar, A. Long-term potentiation at CA3-CA1 hippocampal synapses with special emphasis on aging, disease, and stress. Front. Aging Neurosci. 2011, 3, 1–20.
- Bokun, V.; Moore, J.J.; Moore, R.; Smallcombe, C.C.; Harford, T.J.; Rezaee, F.; Esper, F.; Piedimonte, G. Respiratory syncytial virus exhibits differential tropism for distinct human placental cell types with Hofbauer cells acting as a permissive reservoir for infection. PLoS ONE 2019, 14, e0225767.
- Englund, J.A.; Chu, H.Y. Respiratory Virus Infection During Pregnancy: Does It Matter? J. Infect. Dis. 2018, 218, 512–515.
- Hause, A.M.; Panagiotakopoulos, L.; Weintraub, E.S.; Sy, L.S.; Glenn, S.C.; Tseng, H.-F.; McNeil, M.M. Adverse Outcomes in Pregnant Women Hospitalized With Respiratory Syncytial Virus Infection: A Case Series. Clin. Infect. Dis. 2021, 72, 138–140.
- Manti, S.; Cuppari, C.; Lanzafame, A.; Salpietro, C.; Betta, P.; Leonardi, S.; Perez, M.K.; Piedimonte, G. Detection of respiratory syncytial virus (RSV) at birth in a newborn with respiratory distress. Pediatr. Pulmonol. 2017, 52, E81–E84.
- Shi, L.; Fatemi, S.H.; Sidwell, R.W.; Patterson, P.H. Maternal Influenza Infection Causes Marked Behavioral and Pharmacological Changes in the Offspring. J. Neurosci. 2003, 23, 297–302.
- Quagliato, L.A.; de Matos, U.; Nardi, A.E. Maternal immune activation generates anxiety in offspring: A translational meta-analysis. Transl. Psychiatry 2021, 11, 1–6.
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777.
- Brown, A.S.; Meyer, U. Maternal Immune Activation and Neuropsychiatric Illness: A Translational Research Perspective. Am. J. Psychiatry 2018, 175, 1073–1083.
- Shi, L.; Smith, S.E.P.; Malkova, N.; Tse, D.; Su, Y.; Patterson, P.H. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav. Immun. 2009, 23, 116–123.
- Eisenhut, M. Extrapulmonary manifestations of severe respiratory syncytial virus infection--a systematic review. Crit. Care 2006, 10, R107.
- Somerville, L.K.; Basile, K.; Dwyer, D.E.; Kok, J. The Impact of Influenza Virus Infection in Pregnancy. Future Microbiol. 2018, 13, 263–274.
- Reisinger, S.; Khan, D.; Kong, E.; Berger, A.; Pollak, A.; Pollak, D.D. The Poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol. Ther. 2015, 149, 213–226.
- Moreno, J.L.; Kurita, M.; Holloway, T.; López, J.; Cadagan, R.; Martínez-Sobrido, L.; García-Sastre, A.; González-Maeso, J. Maternal influenza viral infection causes schizophrenia-like alterations of 5-HT2Aand mGlu2 receptors in the adult offspring. J. Neurosci. 2011, 31, 1863–1872.
- Bohmwald, K.; Andrade, C.A.; Kalergis, A.M. Contribution of Pro-Inflammatory Molecules Induced by Respiratory Virus Infections to Neurological Disorders. Pharmaceuticals 2021, 14, 340.
- Kawasaki, Y.; Suyama, K.; Go, H.; Hosoya, M. Clinical manifestations of respiratory syncytial virus-associated encephalopathy in Fukushima, Japan. Pediatr. Int. 2019, 61, 802–806.
- Jiang, Y.; Patel, C.D.; Manivanh, R.; North, B.; Backes, I.M.; Posner, D.A.; Gilli, F.; Pachner, A.R.; Nguyen, L.N.; Leib, D.A. Maternal antiviral immunoglobulin accumulates in neural tissue of neonates to prevent HSV neurological disease. MBio 2017, 8, e00678-17.




