
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gandhi Fernando Pavón-Romero | + 2260 word(s) | 2260 | 2022-01-10 10:32:14 | | | |
| 2 | Beatrix Zheng | + 27 word(s) | 2287 | 2022-01-17 03:22:02 | | |
Video Upload Options
Allergen immunotherapy (AIT) is the sole disease-modifying treatment for allergic rhinitis; it prevents rhinitis from progressing to asthma and lowers medication use. AIT against mites, insect venom, and certain kinds of pollen is effective. The mechanism of action of AIT is based on inducing immunological tolerance characterized by increased IL-10, TGF-β, and IgG4 levels and Treg cell counts. However, AIT requires prolonged schemes of administration and is sometimes associated with adverse reactions. Over the last decade, novel forms of AIT have been developed, focused on better allergen identification, structural modifications to preserve epitopes for B or T cells, post-traductional alteration through chemical processes, and the addition of adjuvants. These modified allergens induce clinical-immunological effects similar to those mentioned above, increasing the tolerance to other related allergens but with fewer side effects. Clinical studies have shown that molecular AIT is efficient in treating grass and birch allergies.
1. Introduction
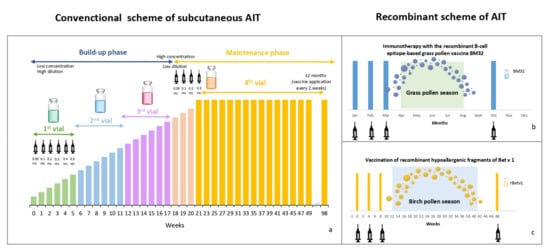
2. Efficacy of Allergen Immunotherapy
3. Clinical-Immune Efficacy of Recombinant Allergens
3.1. Cat
3.2. Birch
3.3. Grasses
4. Conclusions
References
- Ring, J.; Gutermuth, J. 100 years of hyposensitization: History of allergen-specific immunotherapy (ASIT). Allergy 2011, 66, 713–724.
- Frankland, A.W.; Augustin, R. Prophylaxis of summer hay-fever and asthma. Lancet 1954, 263, 1055–1057.
- Bousquet, J.; Schünemann, H.J.; Togias, A.; Bachert, C.; Erhola, M.; Hellings, P.W.; Klimek, L.; Pfaar, O.; Wallace, D.; Ansotegui, I.; et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J. Allergy Clin. Immunol. 2020, 145, 70–80.e3.
- Global Initiative of Asthma. Global Strategy for Asthma Management and Prevention. GINA. 2021. Available online: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf (accessed on 15 September 2021).
- Pfaar, O.; Bachert, C.; Bufe, A.; Buhl, R.; Ebner, C.; Eng, P.; Friedrichs, F.; Fuchs, T.; Hamelmann, E.; Hartwig-Bade, D.; et al. Guideline on allergen-specific immunotherapy in IgE-mediated allergic diseases. Allergo J. Int. 2014, 23, 282–319.
- Durham, S.R.; Walker, S.M.; Varga, E.-M.; Jacobson, M.R.; O’Brien, F.; Noble, W.; Till, S.J.; Hamid, Q.A.; Nouri-Aria, K.T. Long-Term Clinical Efficacy of Grass-Pollen Immunotherapy. N. Engl. J. Med. 1999, 341, 468–475.
- Penagos, M.; Durham, S.R. Duration of allergen immunotherapy for inhalant allergy. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 594–605.
- Pavon-Romero, G.F.; Larenas-Linnemann, D.E.; Xochipa Ruiz, K.K.E.; Ramirez-Jimenez, F.; Teran, L.M.; Ramirez-Jimenez, F.; Teran, L. Subcutaneous Allergen-Specific Immunotherapy Is Safe in Pediatric Patients with Allergic Rhinitis. Int. Arch. Allergy Immunol. 2021, 182, 553–561.
- Larenas-Linnemann, D.; Luna-Pech, J.; Rodríguez-Pérez, N.; Rodríguez-González, M.; Arias-Cruz, A.; Blandón-Vijil, M.; Costa-Domínguez, M.; Del Río-Navarro, B.; Estrada-Cardona, A.; Navarrete-Rodríguez, E.; et al. GUIMIT 2019, Mexican Guideline on Immunotherapy. Guideline on the diagnosis of IgE-mediated allergic disease and immunotherapy following the ADAPTE approach. Rev. Alerg. Mex. 2019, 66 (Suppl. S1), 1–105.
- Virchow, J.C.; Backer, V.; Kuna, P.; Prieto, L.; Nolte, H.; Villesen, H.H.; Ljørring, C.; Riis, B.; De Blay, F. Efficacy of a House Dust Mite Sublingual Allergen Immunotherapy Tablet in Adults With Allergic Asthma. JAMA 2016, 315, 1715–1725.
- Fortescue, R.; Kew, K.M.; Leung, M.S.T. Sublingual immunotherapy for asthma. Cochrane Database Syst. Rev. 2020, 9, CD011293–CD011293.
- Calderón, M.A.; Alves, B.; Jacobson, M.; Hurwitz, B.; Sheikh, A.; Durham, S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst. Rev. 2007, 2007, CD001936.
- Dhami, S.; Nurmatov, U.; Arasi, S.; Khan, T.; Asaria, M.; Zaman, H.; Agarwal, A.; Netuveli, G.; Roberts, G.; Pfaar, O.; et al. Allergen immunotherapy for allergic rhinoconjunctivitis: A systematic review and meta-analysis. Allergy 2017, 72, 1597–1631.
- Coop, C.A. Immunotherapy for Mold Allergy. Clin. Rev. Allergy Immunol. 2013, 47, 289–298.
- Bozek, A.; Pyrkosz, K. Immunotherapy of mold allergy: A review. Hum. Vaccines Immunother. 2017, 13, 2397–2401.
- Radulovic, S.; Calderón, M.A.; Wilson, D.; Durham, S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst. Rev. 2010, 2010, 002893.
- Sieber, J.; Shah-Hosseini, K.; Mösges, R. Specific immunotherapy for allergic rhinitis to grass and tree pollens in daily medical practice—Symptom load with sublingual immunotherapy compared to subcutaneous immunotherapy. Ann. Med. 2011, 43, 418–424.
- Nelson, H.; Cartier, S.; Allen-Ramey, F.; Lawton, S.; Calderon, M.A. Network Meta-analysis Shows Commercialized Subcutaneous and Sublingual Grass Products Have Comparable Efficacy. J. Allergy Clin. Immunol. Pract. 2015, 3, 256–266.e3.
- Dhami, S.; Agarwal, A. Does evidence support the use of cat allergen immunotherapy? Curr. Opin. Allergy Clin. Immunol. 2018, 18, 350–355.
- Calderon, M.A.; Casale, T.B.; Nelson, H.S.; Demoly, P. An evidence-based analysis of house dust mite allergen immunotherapy: A call for more rigorous clinical studies. J. Allergy Clin. Immunol. 2013, 132, 1322–1336.
- Marogna, M.; Spadolini, I.; Massolo, A.; Canonica, G.W.; Passalacqua, G. Long-lasting effects of sublingual immunotherapy according to its duration: A 15-year prospective study. J. Allergy Clin. Immunol. 2010, 126, 969–975.
- Marogna, M.; Bruno, M.; Massolo, A.; Falagiani, P. Long-Lasting Effects of Sublingual Immunotherapy for House Dust Mites in Allergic Rhinitis with Bronchial Hyperreactivity: A Long-Term (13-Year) Retrospective Study in Real Life. Int. Arch. Allergy Immunol. 2006, 142, 70–78.
- Pajno, G.B.; Barberio, G.; De Luca, F.; Morabito, L.; Parmiani, S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin. Exp. Allergy 2001, 31, 1392–1397.
- Inal, A.; Altintas, D.; Yilmaz, M.; Karakoc, G.; Kendirli, S.; Sertdemir, Y. Prevention of new sensitizations by specific immunotherapy in children with rhinitis and/or asthma monosensitized to house dust mite. J. Investig. Allergol. Clin. Immunol. 2007, 17, 85–91.
- Tabar, A.I.; Prieto, L.; Alba, P.; Nieto, A.; Rodríguez, M.; Torrecillas, M.; Huertas, B.; Gómez, E.; Fernández, F.J.; Blanca, M.; et al. Double-blind, randomized, placebo-controlled trial of allergen-specific immunotherapy with the major allergen Alt a 1. J. Allergy Clin. Immunol. 2019, 144, 216–223.e3.
- Soyyigit, S.; Guloglu, D.; Ikinciogullari, A.; Secil, D.; Oztuna, D.; Mungan, D.; Misirligil, Z.; Sin, B.A. Immunologic alterations and efficacy of subcutaneous immunotherapy with Dermatophagoides pteronyssinus in monosensitized and polysensitized patients. Ann. Allergy Asthma Immunol. 2016, 116, 244–251.e2.
- Kucuksezer, U.C.; Ozdemir, C.; Cevhertas, L.; Ogulur, I.; Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy and allergen tolerance. Allergol. Int. 2020, 69, 549–560.
- Özdemir, S.K.; Sin, B.A.; Güloğlu, D.; Ikincioğulları, A.; Gençtürk, Z.; Mısırlıgil, Z. Short-Term Preseasonal Immunotherapy: Is Early Clinical Efficacy Related to the Basophil Response? Int. Arch. Allergy Immunol. 2014, 164, 237–245.
- Epstein, T.G.; Liss, G.M.; Murphy-Berendts, K.; Bernstein, D.I. Risk factors for fatal and nonfatal reactions to subcutaneous immunotherapy. Ann. Allergy Asthma Immunol. 2016, 116, 354–359.e2.
- Bernstein, D.I.; Wanner, M.; Borish, L.; Liss, G.M.; The Immunotherapy Committee of the American Academy of Allergy, Asthma and Immunology. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990–2001. J. Allergy Clin. Immunol. 2004, 113, 1129–1136.
- Casale, T.B.; Busse, W.W.; Kline, J.; Ballas, Z.; Moss, M.H.; Townley, R.G.; Mokhtarani, M.; Seyfert-Margolis, V.; Asare, A.; Bateman, K. Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitis. J. Allergy Clin. Immunol. 2006, 117, 134–140.
- Kuehr, J.; Brauburger, J.; Zielen, S.; Schauer, U.; Kamin, W.; Von Berg, A.; Leupold, W.; Bergmann, K.-C.; Rolinck-Werninghaus, C.; Gräve, M.; et al. Efficacy of combination treatment with anti-IgE plus specific immunotherapy in polysensitized children and adolescents with seasonal allergic rhinitis. J. Allergy Clin. Immunol. 2002, 109, 274–280.
- Kopp, M.V.; Hamelmann, E.; Zielen, S.; Kamin, W.; Bergmann, K.-C.; Sieder, C.; Stenglein, S.; Seyfried, S.; Wahn, U.; for The DUAL Study Group. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co-morbid seasonal allergic asthma. Clin. Exp. Allergy 2009, 39, 271–279.
- Saarne, T.; Neimert-Andersson, T.; Grönlund, H.; Jutel, M.; Gafvelin, G.; van Hage, M. Treatment with a Fel d 1 hypoallergen reduces allergic responses in a mouse model for cat allergy. Allergy 2010, 66, 255–263.
- Klimek, L.; Pfaar, O.; Worm, M. New opportunities for allergen immunotherapy using synthetic peptide immuno-regulatory epitopes (SPIREs). Expert Rev. Clin. Immunol. 2016, 12, 1123–1135.
- Maguirea, P.; Nicodemusb, C.; Robinsonb, D.; Aaronson, D.; Umetsu, D.T. The Safety and Efficacy of ALLERVAX CAT in Cat Allergic Patients. Clin. Immunol. 1999, 93, 222–231.
- Worm, M.; Lee, H.-H.; Kleine-Tebbe, J.; Hafner, R.P.; Laidler, P.; Healey, D.; Buhot, C.; Verhoef, A.; Maillère, B.; Kay, A.B.; et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J. Allergy Clin. Immunol. 2011, 127, 89–97.e14.
- Couroux, P.; Patel, D.; Hafner, R.P.; Armstrong, K.; Larche, M. Fel d 1-derived synthetic peptide immuno-regulatory epitopes show a long-term treatment effect in cat allergic subjects. Clin. Exp. Allergy 2015, 45, 974–981.
- Rudulier, C.D.; Tonti, E.; James, E.; Kwok, W.W.; Larché, M. Modulation of CRTh2 expression on allergen-specific T cells following peptide immunotherapy. Allergy 2019, 74, 2157–2166.
- Senti, G.; Crameri, R.; Kuster, D.; Johansen, P.; Martinez-Gomez, J.M.; Graf, N.; Steiner, M.; Hothorn, L.A.; Grönlund, H.; Tivig, C.; et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J. Allergy Clin. Immunol. 2012, 129, 1290–1296.
- Grönlund, H.; Gafvelin, G. Recombinant Bet v 1 vaccine for treatment of allergy to birch pollen. Hum. Vaccines 2010, 6, 970–977.
- Schülke, S.; Kuttich, K.; Wolfheimer, S.; Duschek, N.; Wangorsch, A.; Reuter, A.; Briza, P.; Pablos, I.; Gadermaier, G.; Ferreira, F.; et al. Conjugation of wildtype and hypoallergenic mugwort allergen Art v 1 to flagellin induces IL-10-DC and suppresses allergen-specific TH2-responses in vivo. Sci. Rep. 2017, 7, 11782.
- Niederberger, V.; Horak, F.; Vrtala, S.; Spitzauer, S.; Krauth, M.-T.; Valent, P.; Reisinger, J.; Pelzmann, M.; Hayek, B.; Kronqvist, M.; et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc. Natl. Acad. Sci. USA 2004, 101, 14677–14682.
- Reisinger, J.; Horak, F.; Pauli, G.; van Hage, M.; Cromwell, O.; König, F.; Valenta, R.; Niederberger, V. Allergen-specific nasal IgG antibodies induced by vaccination with genetically modified allergens are associated with reduced nasal allergen sensitivity. J. Allergy Clin. Immunol. 2005, 116, 347–354.
- Gafvelin, G.; Thunberg, S.; Kronqvist, M.; Grönlund, H.; Grönneberg, R.; Troye-Blomberg, M.; Akdis, M.; Fiebig, H.; Purohit, A.; Horak, F.; et al. Cytokine and Antibody Responses in Birch-Pollen-Allergic Patients Treated with Genetically Modified Derivatives of the Major Birch Pollen Allergen Bet v 1. Int. Arch. Allergy Immunol. 2005, 138, 59–66.
- Campana, R.; Moritz, K.; Neubauer, A.; Huber, H.; Henning, R.; Brodie, T.M.; Kaider, A.; Sallusto, F.; Wöhrl, S.; Valenta, R. Epicutaneous allergen application preferentially boosts specific T cell responses in sensitized patients. Sci. Rep. 2017, 7, 11657.
- Meyer, W.; Narkus, A.; Salapatek, A.M.; Hafner, D. Double-blind, placebo-controlled, dose-ranging study of new recombinant hypoallergenic Bet v 1 in an environmental exposure chamber. Allergy 2013, 68, 724–731.
- Nony, E.; Bouley, J.; Le Mignon, M.; Lemoine, P.; Jain, K.; Horiot, S.; Mascarell, L.; Pallardy, M.; Vincentelli, R.; Leone, P.; et al. Development and evaluation of a sublingual tablet based on recombinant Bet v 1 in birch pollen-allergic patients. Allergy 2015, 70, 795–804.
- Gehlhar, K.; Schlaak, M.; Becker, W.-M.; Bufe, A. Monitoring allergen immunotherapy of pollen-allergic patients: The ratio of allergen-specific IgG4 to IgG1 correlates with clinical outcome. Clin. Exp. Allergy 1999, 29, 497–506.
- Cornelius, C.; Schöneweis, K.; Georgi, F.; Weber, M.; Niederberger, V.; Zieglmayer, P.; Niespodziana, K.; Trauner, M.; Hofer, H.; Urban, S.; et al. Immunotherapy With the PreS-based Grass Pollen Allergy Vaccine BM32 Induces Antibody Responses Protecting Against Hepatitis B Infection. EBioMedicine 2016, 11, 58–67.
- Valenta, R.; Campana, R.; Niederberger, V. Recombinant allergy vaccines based on allergen-derived B cell epitopes. Immunol. Lett. 2017, 189, 19–26.
- Niederberger, V.; Neubauer, A.; Gevaert, P.; Zidarn, M.; Worm, M.; Aberer, W.; Malling, H.J.; Pfaar, O.; Klimek, L.; Pfützner, W.; et al. Safety and efficacy of immunotherapy with the recombinant B-cell epitope–based grass pollen vaccine BM32. J. Allergy Clin. Immunol. 2018, 142, 497–509.e9.
- Zieglmayer, P.; Focke-Tejkl, M.; Schmutz, R.; Lemell, P.; Zieglmayer, R.; Weber, M.; Kiss, R.; Blatt, K.; Valent, P.; Stolz, F.; et al. Mechanisms, safety and efficacy of a B cell epitope-based vaccine for immunotherapy of grass pollen allergy. EBioMedicine 2016, 11, 43–57.
- Eckl-Dorna, J.; Weber, M.; Stanek, V.; Linhart, B.; Ristl, R.; Waltl, E.E.; Merino, S.V.; Hummel, A.; Focke-Tejkl, M.; Froeschel, R.; et al. Two years of treatment with the recombinant grass pollen allergy vaccine BM32 induces a continuously increasing allergen-specific IgG4 response. EBioMedicine 2019, 50, 421–432.
- Rauber, M.M.; Möbs, C.; Campana, R.; Henning, R.; Schulze-Dasbeck, M.; Greene, B.; Focke-Tejkl, M.; Weber, M.; Valenta, R.; Pfützner, W. Allergen immunotherapy with the hypoallergenic B-cell epitope-based vaccine BM32 modifies IL-10- and IL-5-secreting T cells. Allergy 2020, 75, 450–453.
- Martin, J.G.; Panariti, A. Fenotipos del asma, ¿son importantes? Arch. Bronconeumol. Engl. Ed. 2017, 53, 177–179.




