Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ana LUis | + 1808 word(s) | 1808 | 2022-01-05 02:54:15 | | | |
| 2 | Catherine Yang | Meta information modification | 1808 | 2022-01-14 02:09:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Luis, A. Extremely Acidic Eukaryotic (Micro) Organisms. Encyclopedia. Available online: https://encyclopedia.pub/entry/18194 (accessed on 07 February 2026).
Luis A. Extremely Acidic Eukaryotic (Micro) Organisms. Encyclopedia. Available at: https://encyclopedia.pub/entry/18194. Accessed February 07, 2026.
Luis, Ana. "Extremely Acidic Eukaryotic (Micro) Organisms" Encyclopedia, https://encyclopedia.pub/entry/18194 (accessed February 07, 2026).
Luis, A. (2022, January 13). Extremely Acidic Eukaryotic (Micro) Organisms. In Encyclopedia. https://encyclopedia.pub/entry/18194
Luis, Ana. "Extremely Acidic Eukaryotic (Micro) Organisms." Encyclopedia. Web. 13 January, 2022.
Copy Citation
Acid Mine Drainage (AMD) results from sulfide oxidation, which incorporates hydrogen ions, sulfate, and metals/metalloids into the aquatic environment, allowing fixation, bioaccumulation and biomagnification of pollutants in the aquatic food chain. Acidic leachates from waste rock dams from pyritic and (to a lesser extent) coal mining are the main foci of Acid Mine Drainage (AMD) production.
: AMD (Acid Mine Drainage)
metal mining
1. Diatoms
The diatoms are one of the most effective ecological indicators [1][2][3][4][5][6][7] in AMD-contaminated environments, due to their ubiquity in aquatic habitats [8] and high effectiveness for assessing aquatic health [9]. Thus, they are good indicators of pH changes and very abundant in environments impacted by low pH [10]. Diatoms respond to chemical stress at community and individual levels. At a community level, the highest metal concentrations (i.e., Fe: 6 g/L, Zn 1.7 g/L, Cu 347 mg/L, Cd 3.5 mg/L, Ni 3 mg/L, Mn 0.3 mg/L) and low pH (i.e., 2.0-4.5) result in low diatom diversity (Shannon–Winer diversity index < 2.2 on a 5-point scale) [1][2][3], and the species change to more acidophilic or acidobiontic varieties better-prepared to endure these harsh conditions. This decrease in species richness has been observed in many works [11][2][3][5][6][7][12][13], and is more prominent for diatoms than for macroinvertebrates [14].
The dominant and typical species in acidic waters are Pinnularia acoricola, Pinnularia acidophila, Pinnularia aljustrelica, Eunotia exigua (Figure 1) and Nitzschia hantzschiana [11][1][2][6]. The three Pinnularia species found in the impacted sites; P. aljustrelica is the most abundant due to its capacity to survive a very low pH, i.e., 1.9–4.2 [2][15]. Achnanthidium minutissimum is a difficult species, able to tolerate different environmental conditions and usually the only Achnanthidium species reported in AMD polluted streams [16], being abundant in a wide variety of habitats and environmental conditions [17]. However, A. minutissimum can also appear in unimpacted sites, being the dominant species in less-impacted sites [1]. It is considered to generally be the first taxon to colonize different habitats (e.g., rocks, sediments) [18], and has the ability to invade open areas following changes in environmental conditions [19].
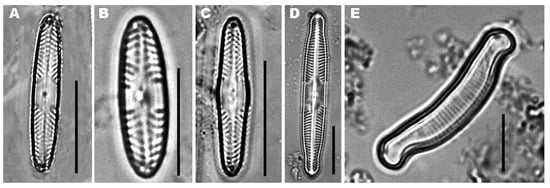
Figure 1. (A) Pinnularia acidophila, (B) Pinnularia acoricola, (C) Pinnularia aljustrelica, (D) Pinnularia subcapitata and (E) Eunotia exigua.
In the Lousal and Aljustrel mining areas located in the Portuguese part of the IPB, the species found (in descending order of dominance) include Brachysira vitrea, Eunotia exigua and Pinnularia c.f. acidophila (Figure 1). In the Aljustrel mining area, with sulfated high to extreme metal/metalloid concentrations and low pH waters, P. aljustrelica, E. exigua (Figure 1) and Nitzschia aff. hantzschiana are the dominant species [2]. However, E. exigua is an acidobiontic taxon, and is the most widespread species in AMD-contaminated streams such as the Río Tinto [3][6][20][21][22] and the Aljustrel streams [1][2][4][23] (Table 1).
Table 1. Diatom species with pH and metal concentrations (mg/L), pH tolerance range and optimum pH.
| Species Name | pH Tolerance Range | Optimum pH | Metal Concentrations |
|---|---|---|---|
| Pinnularia aljustrelica | 2.0–5.0 | 2.0–3.0 | Fe 1300 to 6000 Cu 230–350 Zn 118–170 |
| Pinnularia acidophila | 2.0–4.5 | 2.0–2.2 | |
| Pinnularia acoricola | 2.0–6.0 | 2.0–3.0 | |
| Nitzschia thermalis | 2.0–7.0 | 3.0 | |
| Nitzschia hantzschiana | 2.0–6.8 | 2.0–2.2 | |
| Eunotia exigua | 3.0–5.0 | 3.0 | Similar metal concentrations as above, but species valves are morphologically affected by metals (teratologies) |
| Brachysira vitrea | 4.5–7.5 | 4.8 | Fe 1100 Zn 0.30 Cu 0.64 |
Metals lower biodiversity in several important ways. Diatoms have developed mechanisms such as biotransformation, biomineralization, bioaccumulation and biosorption to cope with heavy metal toxicity [24]; nevertheless, pollution-tolerant and pollution-sensitive diatoms have different responses to metal pollution [25]. When exposed to metals, community size can be impaired through reduction of cell number, selection for smaller species, and decrease in cell size within a given species [4][26][27][28]; diatom growth can be delayed or inhibited, therefore reducing diatom biomass [29] and decreasing the rates of survival and growth. Diatoms are able to sequestrate large quantities of metals from waters [30]. The most common taxa presenting abnormal valves due to metals/pH or metal-pH combination are Fragilaria capucina [31], Fragilaria rumpens and A. minutissimum [31] and Eunotia exigua [2].
Thus, the observed differences in diatom community structure result from the combined action of low pH and highly soluble heavy metals [32][33]. Diatoms can also be susceptible at the individual level showing changes in frustule morphology [4]. The resistance of A. minutissimum to metals is still under discussion, with contradictory results in the literature. It is usually considered an indicator of metal pollution [34], although it could also indicate good general water quality [35].
2. Unicellular and Filamentous Green Algae
Although AMD environments are not appetizing to many species, some genera of unicellular and filamentous green algae can adapt and survive; among these are species from the unicellular genera Chlamydomonas, Chlorella, Cyanidium, Dunaliella, Euglena [36][37] and from the filamentous genera Klesormidium, Microspora, Mougeotia, Ulothrix, Stigeoclomium, Zygnema and Microthammion. The genera Mougeotia, Ulothrix, Chlamydomonas, Chara and Nitella are typical of these environments; however, they may not be as abundant as diatoms [38][39][40].
Cyanidium is a red algae genus, or rhodophite. It has been observed at pH 1.2–1.8 in waters close to the Rio Tinto mines. Dunaliella, Chlamydomonas and Chlorella are unicellular green algae from the Chlorophyceae family. Both Chlamydomonas and Dunaliella may be motile, with the presence of flagella. Curiously, Dunaliella has no cell wall. Chlamydomonas acidophila is the most abundant species in acid waters, showing a high tolerance to copper and other heavy metals [41][42] Euglena mutabilis is abundant in shallow waters and easily forms large tufts that can look like filamentous algae. Oxygen bubbles are frequently observed in some places where Euglena thrives. All microalgae contribute to enhanced oxygen production (up to 200% saturation) and organic carbon, which reduces the oligotrophic conditions of AMD-polluted waters and increases the oxidative activity of aerobic chemoautolithotrophic bacteria and heterotrophic bacteria [43].
The acidophilic species of the Mougeotia genus can survive in the AMD environment, in waters with a pH of 2.9–4.1 [44]. The abundance and distribution of Klebsormidium sp. in AMD affected waters makes this species a good ecological indicator of this type of contamination, and Klebsormidium-dominated algal mats are particularly good indicators of high iron concentrations in water [45]. Additionally, Mougeotia, can be abundant in AMD streams [46][45], possibly because of strong competition for low DIC (dissolved inorganic carbon) in acidic environments [47]. The genus Klebsormidium is known to be metal resistant, and is been related with metal-rich polluted waters. K. subtile, K. rivulare, K. flaccidium and K. acidophilum are other species related with AMD-contaminated environments [46][17][40]. Chlamydomonas sp. shows tolerance in a wide range of physical and chemical conditions in a lake contaminated by AMD, being consistently present [48].
The Microspora genus is very abundant in mines with high levels of metal pollution, and is considered by [49] as a good bioindicator. The Ulothrix genus, on the other hand, is predominant in biofilms from AMD-contaminated sites, having a great capacity to recover Cu and As.
Several of the microorganisms described above are represented in Figure 1.
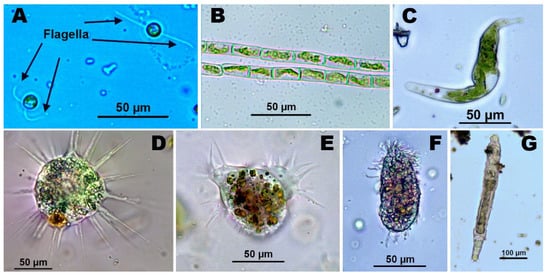
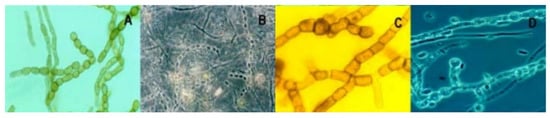
Figure 1. (A) Unicellular algae (Chlamydomonas acidophila), (B) Filamentous algae (Klebsormidium sp.), (C) Euglena mutabilis, (D) Protozoo Heliozoa, (E) Protozoo Ciliata, (F) Amoeba, (G) Rotífer.
3. Protozoa, Fungi and Yeasts in AMD-Polluted Waters
In AMD-polluted waters, several groups of heterotrophic protists may be observed. The main groups include protozoa: ciliates such as Urotricha and Oxytricha, flagellates such as Bodo and Ochromonas, amoebas such as Actinophyrs and Naegleria, etc., and heliozoa. In acidic waters, these genera play an essential role in nutrient recycling in spite of their oligotrophic characteristics [50].
Fungi are more acidotolerant than acidophilic, although some filamentous fungi, such as as Acontium, Cephalosporium and the yeast Trichosporon, are able to growth up to pH 0 [50]. In [51], a wide variety of filamentous fungi are described, including Scytalidium, Bahusakala, Phoma, Heteroconium, and even Penicillium and diverse ascomycetes and zygomycetes. In addition to their role as components of an acidic river ecosystem, fungi play an important role in the biomineralization of iron and the accumulation of intracellular deposits of toxic metals [52][53]; see examples below (Figure 2).
4. The Impact of AMD on Micro-Macroinvertebrates
AMD represents an extremely stressful and long-term source of pollution due to the anthropogenic disturbance of geological layers. Characteristic low pH and high metal concentrations have been highlighted as the main drivers of micro- (<500 µm length) and macroinvertebrate (>500 µm, length) diversity and community composition in streams affected by AMD [55][5][56], while acidification may induce an increase in the bioaccumulation of metals in insect larvae with consequences for the food chain and aquatic fauna [57]. The main microinvertebrates observed in these waters are from phylum Rotifera, considered pseudocoelomate “animals” [54][58][59].
Variation in macroinvertebrate assemblages and densities has also shown a strong relationship with other water chemistry variables in addition to metals, such as dissolved oxygen and conductivity, inducing a clear shift from metal-sensitive (e.g., Ephemeroptera, Plecoptera and Trichoptera) to metal-tolerant (Diptera, Coleoptera and Collembolla) taxa [60][61][62][63][64]. The order Ephemeroptera is a group highly sensitive to metals; however, some species, such as Baetis rhodani and Caenis cf. luctuosa, exhibit tolerance to these contaminants [65][66].
Among metal-tolerant taxa, Chironomidae (Diptera) assemblages often represent a significant portion of the sediment-dwelling fauna at deteriorated sites, and are hence especially useful as bioindicators and for sediment quality assessment [67][68][69]. Chironomid species have been found in acidified metal-polluted temperate [69][70], tropical and high-altitude streams [62][71] as well as unpolluted glacier-elevated water streams [72]. Species of Chironomus may have physiological adaptations responsible for such tolerance, as those species coming from contaminated points are able to adjust their body metals concentration when compared to other species [63]. Chironomids from elevated altitudes and metal-contaminated sites contain more melanin than species from reference sites at lower altitudes [73]. This fact highlights the importance of melanin in chironomids as a UV-B radiation protector and metal chelator. In addition, genetic adaptation has been found to be a metal tolerance tool in Chironomus species from highly contaminated environments [74][75][76]. In [63], it was found that only one tolerant strain of chironomids was able to survive in the most metal-rich points in the Andes, which indicates that tolerance could have been developed as an answer to naturally existing acid and metal-rich environments, and thus may have preceded human-influenced alterations due to mining activity. The adaptation of this unique chironomid species to very large metal values may have come with direct costs, as represented by smaller specimens in comparison to those from species in similar reference streams, in the form of reallocation of energy towards resistance tools such as metal-binding metallothioneins. Melanin production or cuticle sclerotization in chironomids [73] may convey a trade-off evidenced as reduced growth [77].
Most taxa within the Chironomidae (Figure 3) are collector-filterers and collector-gatherers while a few (e.g., Cryptochironomus sp., Endochironomus spp., Glyptotendipes spp., Polypedilum spp. and Chironomus spp.) are predatory on oligochaetes in AMD-contaminated sites [66], which indicates that these group show different ecological response patterns to AMD [69]. Moreover, shredder-climbers can be the dominant group at impacted sites and could be more adaptive in AMD affected streams than other groups, as Fe-loving bacteria growing on leaves coated with Fe hydroxide become an option as a food resource [78].
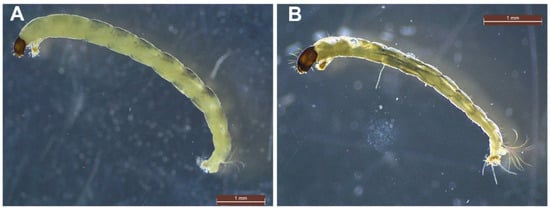
Figure 3. Microphotographs of individuals from family Chironomidae: (A) Cricotopus sp., (B) subfamily Orthocladinae.
In general, stress conditions may benefit the increase of secondary consumers, changing, considerably, the food chain shape [69]. This phenomenon has been described for macroinvertebrates from AMD impacted streams [66] and implies major shifts in resource utilisation, possibly reducing the number of trophic levels and consequently simplifying the food web. While these ecological processes still need further analysis in AMD environments, they can explain the use of Tanypodinae as bioindicators. Thus, AMD contamination sites can have high biodiversity because of high tolerant species richness, as well as considerable variability in metal tolerance among macroinvertebrate taxa and species (Byrne et al., 2012). When compared to reference sites, the functional diversity of macroinvertebrates is lessened, and their functional structure is much simpler [79].
References
- Luís, A.T.; Teixeira, P.; Almeida, S.F.P.; Ector, L.; Matos, J.X.; Ferreira da Silva, E.A. Impact of acid mine drainage (AMD) on water quality, stream sediments and periphytic diatom communities in the surrounding streams of Aljustrel mining area (Portugal). Water Air Soil Pollut. 2009, 200, 147–167.
- Luís, A.T.; Durães, N.; Almeida, S.F.P.; Ferreira da Silva, E.A. Integrating geochemical (surface waters, stream sediments) and biological (diatoms) approaches to assess AMD environmental impact in a pyritic mining area: Aljustrel (Alentejo, Portugal. J. Environ. Sci. 2016, 42, 215–226.
- Valente, T.; Rivera, M.J.; Almeida, S.F.P.; Delgado, C.; Gomes, P.; Grande, J.A.; de la Torre, M.L. Characterization of water reservoirs affected by acid mine drainage: Geochemical, mineralogical and biological (diatoms) properties of the water. Environ. Sci. Pollut. Res. Int. 2016, 23, 6002–6011.
- Luís, A.T.; Teixeira, P.; Almeida, S.F.P.; Matos, J.X.; Ferreira da Silva, E. Environmental impact of mining activities in the Lousal area (Portugal): Chemical and diatom characterization of metal-contaminated stream sediments and surface water of Corona stream. Sci. Total Environ. 2011, 409, 4312–4325.
- DeNicola, D.M. A review of diatoms found in highly acidic environments. Hydrobiologia 2000, 433, 111–122.
- Rivera, M.J.; Luís, A.T.; Grande, J.A.; Sarmiento, A.M.; Dávila, J.M.; Fortes, J.C.; Córdoba, F.; Diaz-Curiel, J.; Santisteban, M. Physico-Chemical Influence of Surface Water Contaminated by Acid Mine Drainage on the Populations of Diatoms in Dams (Iberian Pyrite Belt, SW Spain). Int. J. Environ. Res. Public Health 2019, 16, 4516.
- Luís, A.T.; Teixeira, M.; Durães, N.; Pinto, R.; Almeida, S.F.P.; Ferreira da Silva, E.A.; Figueira, E. Extremely acidic environment: Biogeochemical effects on algal biofilms. Ecotoxicol. Environ. Saf. 2019, 177, 124–132.
- Gray, J.B.; Vis, M.L. Reference diatom assemblage response to restoration of an acid mine drainage stream. Ecol. Indic. 2013, 29, 234–245.
- Hill, B.H.; Herlihy, A.T.; Kaufmann, P.R.; Stevenson, R.J.; McCormick, F.H.; Johnson, C.B. Use of periphyton assemblage data as an index of biotic integrity. J. N. Am. Benthol. Soc. 2000, 19, 50–67.
- Zalack, J.T.; Smucker, N.J.; Vis, M.L. Development of a Diatom Index of biotic integrity for acid mine drainage impacted streams. Ecol. Indic. 2010, 10, 287–295.
- Luís, A.T.; Coelho, H.; Almeida, S.F.P.; Ferreira da Silva, E.A.; Serôdio, J. Photosynthetic activity and ecology of benthic diatom communities from streams affected by Acid Mine Drainage (AMD) in pyritic mines. Fundam. Appl. Limnol. 2013, 182, 47–59.
- Smucker, N.J.; Vis, M.L. Use of diatoms to assess agricultural and coal mining impacts on streams and a multiassemblage case study. J. N. Am. Benthol. Soc. 2009, 28, 659–675.
- Van Dam, H.; Merten, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from The Netherlands. Netherland J. Aquat. Ecol. 1994, 28, 117–133.
- Bisthoven, J.L.; Gerhardt, A.; Soares, A.M.V.M. Chironomidae as bioindicators of an acid mine drainage in S. Portugal. Hydrobiologia 2005, 532, 181–191.
- Luís, A.T.; Novais, M.H.; Van de Vijver, B.; Almeida, S.F.P.; Ferreira da Silva, E.A.; Hoffmann, L.; Ector, L. Pinnularia aljustrelica sp. nov. (Bacillariophyceae), a new diatom species found in acidic waters in the Aljustrel mining area (Portugal), and further observations on the taxonomy, morphology and ecology of P. acidophila HOFMANN et KRAMMER and P. acoricola HUSTEDT. Fottea 2012, 12, 27–40.
- Ponader, K.C.; Potapova, M.G. Diatoms from the genus Achnanthidium in flowing waters of the Appalachian Mountains (North America): Ecology, distribution and taxonomic notes. Limnologica 2007, 37, 227–241.
- Verb, R.G.; Vis, M.L. Comparison of benthic diatom assemblages from streams draining abandoned and reclaimed coal mines and nonimpacted sites. J. N. Am. Benthol. Soc. 2000, 19, 274–288.
- Sabater, S. Diatom communities as indicators of environmental stress in the Guadiamar River, S.-W. Spain, following a major mine tailings spill. J. Appl. Phycol. 2000, 12, 113–124.
- Peterson, C.G.; Stevenson, R.J. Resistance and resilience of lotic algal communities: Importance of disturbance timing and current. Ecology 1992, 73, 1445–1461.
- Urrea-Clos, G.; Sabater, S. Comparative study of algal communities in acid and alkaline waters from Tinto, Odiel and Piedras river basins (SW Spain). Limnetica 2009, 28, 261–272.
- Aguilera, A. Eukaryotic organisms in extreme acid environments. Life 2013, 3, 363–374.
- Rivera, M.J.; Santisteban, M.; Aroba, J.; Grande, J.A.; Dávila, J.M.; Sarmiento, A.M.; Fortes, J.C.; Diaz-Curiel, J.; Luís, A.T. Application of Fuzzy Logic Techniques for Biogeochemical Characterization of Dams Affected by Acid Mine Drainage (AMD) Processes in the Iberian Pyrite Belt (IPB), Spain. Water Air Soil Pollut. 2020, 231, 142.
- Luís, A.T.; Grande, J.A.; Dávila, J.M.; Aroba, J.; Durães, N.; Almeida, S.F.P.; de la Torre, M.L.; Sarmiento, A.M.; Fortes, J.C.; Ferreira da Silva, E.; et al. Application of fuzzy logic tools for the biogeochemical characterisation of (un)contaminated waters from Aljustrel mining area (South Portugal). Chemosphere 2018, 211, 736–744.
- Tiwari, A.; Marella, T.K. Potential and application of diatoms for industry-specific wastewater treatment. In Application of Microalgae in Wastewater Treatment; Gupta, S., Bux, F., Eds.; Springer: Cham, Germany, 2019; pp. 321–339.
- Marella, T.K.; Saxena, A.; Tiwari, A. Diatom mediated heavy metal remediation: A review. Bioresour. Technol. 2020, 305, 123068.
- Morin, S.; Vivas-Nogues, M.; Duong, T.T.; Boudou, A.; Coste, M.; Delmas, F. Dynamics of benthic diatom colonization in a cadmium/zinc-polluted river (Riou-Mort, France). Fundam. Appl. Limnol. 2007, 168, 179–187.
- Morin, S.; Cordonier, A.; Lavoie, I.; Arini, A.; Blanco, S.; Duong, T.T.; Tornés, E.; Bonet, B.; Corcoll, N.; Faggiano, L.; et al. Consistency in diatom response to metal-contaminated environments. In Handbook of Environmental Chemistry, Emerging and Priority Pollutants in Rivers; Guasch, H., Ginebreda, A., Geiszinger, A., Eds.; Springer: Heidelberg, Germany, 2012; pp. 117–146.
- Falasco, E.; Bona, F.; Badino, G.; Hoffmann, L.; Ector, L. Diatom teratological forms and environmental alterations: A review. Hydrobiologia 2009, 623, 1–35.
- Gold, C.; Feurtet-Mazel, A.; Coste, M.; Boudou, A. Effects of cadmium stress on periphytic diatom communities in indoor artificial streams. Freshw. Biol. 2003, 48, 316–328.
- Hernández-Ávila, J.; Salinas-Rodríguez, E.; Cerecedo-Sáenz, E.; Reyes-Valderrama, I.; Arenas-Flores, A.; Román-Gutiérrez, A.D.; Rodríguez-Lugo, V. Diatoms and their capability for heavy metal removal by cationic exchange. Metals 2017, 7, 169.
- Ferreira da Silva, E.F.; Almeida, S.F.P.; Nunes, M.L.; Luís, A.T.; Borg, F.; Hedlund, M.; Marques de Sá, C.; Patinha, C.; Teixeira, P. Heavy metal pollution downstream the abandoned Coval da Mó mine (Portugal) and associated effects on epilithic diatom communities. Sci. Total Environ. 2009, 407, 5620–5636.
- Gerhardt, A.; Bisthoven, L.J.; Guhr, K.; Soares, A.M.V.M.; Pereira, M.J. Phytoassessment of acid mine drainage: Lemna gibba bioassay and diatom community structure. Ecotoxicology 2008, 17, 47–58.
- Stewart, P.M.; Smith, E.P.; Cairns-Jr, J. Relationship of the physicochemical environment to diatom and protozoan communities: A multivariate approach. Arch. Für Protistenkd. 1987, 134, 331–341.
- Cattaneo, A.; Couillard, Y.; Wunsam, S.; Courcelles, M. Diatom taxonomic and morphological changes as indicators of metal pollution and recovery in Lac Dufault (Québec, Canada). J. Paleolimnol. 2004, 32, 163–175.
- Coste, M.; Boutry, S.; Tison-Rosebery, J.; Delmas, F. Improvements of the Biological Diatom Index (BDI): Description and efficiency of the new version (BDI-2006). Ecol. Indic. 2009, 9, 621–650.
- Amaral-Zettler, L.A.; Gomez, F.; Zettler, E.; Keenan, B.G.; Amils, R.; Sogin, M.L. Eukaryotic diversity in Spain’s River of Fire. Nature 2002, 417, 137.
- Aguilera, A.; Souza-Egipsy, V.; Gonzalez-Toril, E. La vida en Río Tinto; Centro de Astrobiología-CSIC-INTA, Ministerio de Defensa: Madrid, Spain, 2000; p. 100.
- Verb, R.G.; Vis, M.L. Macroalgal communities from an acid mine drainage impacted watershed. Aquat. Bot. 2001, 71, 93–107.
- Niyogi, D.K.; Lewis, W.M., Jr.; McKnight, D.M. Effects of stress from mine drainage on diversity, biomass, and function of primary producers in mountain streams. Ecosystems 2002, 5, 554–567.
- Novis, P.M. Taxonomy of Klebsormidium (Klebsormidiales, Charophyceae) in New Zealand streams and the significance of low-pH habitats. Phycologia 2006, 45, 293–301.
- Dean, A.P.; Hartley, A.; McIntosh, O.A.; Smith, A.; Feord, H.K.; Holmberg, N.H.; King, T.; Yardley, E.; White, K.N.; Pittman, J.K. Metabolic adaptation of a Chlamydomonas acidophila strain isolated from acid mine drainage ponds with low eukaryotic diversity. Sci. Total Environ. 2019, 647, 75–87.
- Díaz, S.; de Francisco, P.; Olsson, S.; Aguilera, A.; González-Toril, E.; Martín, A.M. Toxicity, physiological, and ultrastructural effects of Arsenic and Cadmium on the extremophilic microalga Chlamydomonas acidophila. Int. J. Environ. Res. Public Health 2020, 17, 1650.
- Aguilera, A.; Suominen, S.; Pétursdóttir, S.; Olgudóttir, E.; Guðmundsdóttir, E.E.; Altamirano, M.; González-Toril, E.; Hreggviðsson, G.O. Physiological plasticity of high-temperature intertidal cyanobacterial microbial mats to temperature and salinity: Daily and seasonal in situ photosynthetic performance. Eur. J. Phycol. 2020, 55, 223–233.
- Freitas, A.P.P.; Schneider, I.A.H.; Schwartzbold, A. Biosorption of heavy metals by algal communities in water streams affected by the acid mine drainage in the coal-mining region of Santa Catarina state. Brazil Miner. Eng. 2011, 24, 1215–1218.
- Stevens, A.E.; McCathy, B.C.; Vis, M.L. Metal content of Klebsormidium-dominated (Chlorophyta) algal mats from acid mine drainage waters in southeastern. J. Torrey Bot. Soc. 2001, 128, 226–233.
- Sabater, S.; Buchaca, T.; Cambra, J.; Catalan, J.; Guasch, H.; Ivorra, N.; Munoz, I.; Navarro, E.; Real, M.; Romaní, A. Structure and function of benthic algal communities in an extremely acid river. J. Phycol. 2003, 39, 481–489.
- Vinebrooke, R.D. Abiotic and biotic regulation of periphyton in recovering acidified lakes. J. N. Am. Benthol. Soc. 1996, 15, 318–331.
- Kalin, M.; Wheeler, W.N.; Olaveson, M.M. Response of phytoplankton to ecological engineering remediation of a Canadian Shield Lake affected by acid mine drainage. Ecol. Eng. 2006, 28, 296–310.
- Novis, P.M. A taxonomic survey of microspora (Chlorophyceae, Chlorophyta) in New Zealand. N. Z. J. Bot. 2004, 42, 153–165.
- Aguilera, A.; González-Toril, E. Eukaryotic life in extreme environments: Acidophilic fungi. In Fungi in Extreme Environments: Ecological Role and Biotechnological Significance; Tiquia-Arashiro, S.M., Grube, M., Eds.; Springer: NY city, NY, USA, 2019; pp. 21–38.
- López-Archilla, A.; González, A.E.; Terrón, M.C.; Amils, R. Ecological study of the fungal populations of the acidic Tinto River in Southwestern Spain. Can. J. Microbiol. 2004, 50, 923–934.
- Duran, C.; Marin, I.; Amils, R. Specific metal sequestering acidophilic fungi. In Biohydrometallurgy and the Environment; Amils, R., Ballester, A., Eds.; Towards the Mining of the 21st Century, Proc. Int. Biohydrometal Symp; Elsevier: Amsterdam, The Netherlands, 1999; pp. 521–530.
- Oggerin, M.; Tornos, F.; Rodríguez, N.; del Moral, C.; Sánchez-Román, M.; Amils, R. Specific jarosite biomineralization by Purpureocillium lilacinum, an acidophilic fungus isolated from Río Tinto. Environ. Microbiol. 2013, 15, 2228–2237.
- López-Archilla, A.I. Rio Tinto: Un universo de mundos microbianos. Ecosistemas 2005, 14, 52–65.
- Clapcott, J.E.; Goodwin, E.O.; Harding, J.S. Identifying catchment-scale predictors of coal mining impacts on New Zealand stream communities. Environ. Manag. 2016, 57, 711–721.
- Wright, I.A.; Paciuszkiewicz, K.; Belmer, N. Increased Water Pollution After Closure of Australia’s Longest Operating Underground Coal Mine: A 13-Month Study of Mine Drainage, Water Chemistry and River Ecology. Water Air Soil Pollut. 2018, 229, 55.
- St. Louiss, V.L. Element concentrations in chironomids and their abundance in the littoral zone of acidified lakes in Northwestern Ontario. J. Fish. Aquat. Sci. 1993, 50, 953–963.
- Deneke, R. Review of rotifers and crustaceans in highly acidic environments of pH values < 3. Hydrobiologia 2000, 433, 167–172.
- Amaral-Zettler, L.A. Eukaryotic diversity at pH extremes. Front. Microbiol. 2013, 3, 1–17.
- Gower, A.M.; Myers, G.; Kent, M.; Foulkes, M.E. Relationships between macroinvertebrate communities and environmental variables in metal-contaminated streams in south-West England. Freshw. Biol 1994, 32, 199–221.
- de Jonge, M.; de Vijuer, B.V.; Blust, R.; Bervoets, L. Responses of aquatic organisms to metal pollution in a lowland river in Flanders: A comparison of diatoms and macroinvertebrates. Sci. Total Environ. 2008, 407, 615–629.
- Loayza-Muro, R.A.; Elias-Letts, R.; Marticorena-Ruiz, J.K.; Palomino, E.J.; Duivenvoorden, J.F.; Kraak, M.H.S.; Admiraal, W. Metal-induced shifts in benthic macroinvertebrate community composition in Andean high altitude streams. Environ. Toxicol. Chem. 2010, 29, 2761–2768.
- Loayza-Muro, R.A.; de Baat, M.L.; Palomino, E.J.; Kuperus, P.; Kraak, M.H.S.; Admiraal, W.; Breeuwer, J.A.J. Metals and altitude drive genetic diversity of chironomids in Andean streams. Freshw. Biol. 2014, 59, 56–63.
- Loayza-Muro, R.A.; Duivenvoorden, J.F.; Kraak, M.H.S.; Admiraal, W. 2014b Metal leaching, acidity, and altitude confine benthic macroinvertebrate community composition in Andean streams. Environ. Toxicol. Chem. 2014, 33, 404–411.
- Beltman, D.J.; Clements, W.H.; Lipton, J.; Cacela, D. Benthic invertebrate metals exposure, accumulation and community-level effects downstream from a hard rock mine site. Environ. Toxicol. Chem. 1999, 18, 299–307.
- Gerhardt, A.L.; Bisthoven, J.; Soares, A.M.V.M. Macroinvertebrate response to acid mine drainage: Community metrics and on-line behavioural toxicity bioassay. Environ. Pollut. 2004, 130, 263–274.
- Canfield, T.J.; Kemble, N.E.; Brumbaugh, W.G.; Dwyer, F.J.; Ingersoll, C.G.; Fairchild, J.F. Use of benthic invertebrate community structure and the sediment quality triad to evaluate metal-contaminated sediment in the upper Clark Fork River, Montana. Environ. Toxicol. Chem. 1994, 13, 1999–2012.
- Bisthoven, L.J.; Gerhardt, A. Chironomidae (Diptera, Nematocera) fauna in three small streams of Skania, Sweden. Environ. Monit. Assess. 2003, 83, 89–102.
- Bisthoven, J.L.; Gerhardt, A.; Soares, A.M.V.M. Effects of Acid Mine Drainage on larval Chironomus (Diptera, Chironomidae) measured with the Multispecies Freshwater Biomonitor. Environ. Toxicol. Chem. 2004, 23, 1123–1128.
- De Haas, E.M.; van Haaren, R.; Koelmans, A.A.; Kraak, M.H.S.; Admiraal, W. Analyzing the causes for the persistence of chironomids in floodplain lake sediments. Arch. Hydrobiol. 2005, 162, 211–228.
- Löhr, A.J.; Sluik, R.; Olaveson, M.M.; Ivorra, N.; van Gestel, C.A.M.; van Straalen, N.M. Macroinvertebrate and algal communities in an extremely acidic river and the Kawah Ijen crater lake (pH < 0.3), Indonesia. Arch. Hydrobiol. 2006, 165, 1–21.
- Hamerlík, L.; Jacobsen, D. Chironomid (Diptera) distribution and diversity in Tibetan streams with different glacial influence. Insect Conserv. Divers. 2011, 5, 319–326.
- Loayza-Muro, R.A.; Marticorena-Ruiz, J.K.; Palomino, E.J.; Merritt, C.; De Baat, M.L.; van Gemert, M.; Verweij, R.A.; Kraak, M.H.S.; Admiraal, W. Persistence of chironomids in metal polluted Andean high altitude streams: Does melanin play a role? Environ. Sci. Technol. 2013, 47, 601–607.
- Groenendijk, D.; Lücker, S.M.G.; Plans, M.; Kraak, M.H.S.; Admiraal, W. Dynamics of metal adaptation in riverine chironomids. Environ. Pollut. 2002, 117, 101–109.
- Van Straalen, N.M.; Donker, M.H.; Vijver, M.G.; van Gestel, C.A.M. Bioavailability of contaminants estimated from uptake rates into soil invertebrates. Environ. Pollut. 2005, 136, 409–417.
- Buchwalter, D.B.; Cain, D.J.; Martin, C.A.; Xie, L.; Luoma, S.N.; Garland, T. Aquatic insect ecophysiological traits reveal phylogenetically based differences in dissolved cadmium susceptibility. Proc. Natl. Acad. Sci. USA 2008, 105, 8321–8326.
- Sibly, R.M.; Calow, P. A life-cycle theory of responses to stress. Biol. J. Linn. Soc. 1989, 37, 101–116.
- Schlief, J.; Mutz, M. Palatability of leaves conditioned in streams affected by mine drainage: A feeding experiment with Gammarus pulex (L.). Hydrobiologia 2006, 563, 445–452.
- He, F.; Jiang, W.; Tang, T.; Cai, Q. Assessing impact of acid mine drainage on benthic macroinvertebrates: Can functional diversity metrics be used as indicators? J. Freshw. Ecol. 2015, 30, 513–524.
More
Information
Subjects:
Environmental Sciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
14 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No