Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sawit Na songkhla | + 1422 word(s) | 1422 | 2021-12-15 07:23:45 | | | |
| 2 | Lindsay Dong | + 971 word(s) | 2393 | 2022-01-13 02:32:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Na Songkhla, S. Applications of Quartz Crystal Microbalance. Encyclopedia. Available online: https://encyclopedia.pub/entry/18165 (accessed on 07 February 2026).
Na Songkhla S. Applications of Quartz Crystal Microbalance. Encyclopedia. Available at: https://encyclopedia.pub/entry/18165. Accessed February 07, 2026.
Na Songkhla, Sawit. "Applications of Quartz Crystal Microbalance" Encyclopedia, https://encyclopedia.pub/entry/18165 (accessed February 07, 2026).
Na Songkhla, S. (2022, January 12). Applications of Quartz Crystal Microbalance. In Encyclopedia. https://encyclopedia.pub/entry/18165
Na Songkhla, Sawit. "Applications of Quartz Crystal Microbalance." Encyclopedia. Web. 12 January, 2022.
Copy Citation
Quartz Crystal Microbalance (QCM) is one of the many acoustic transducers. It is the most popular and widely used acoustic transducer for sensor applications. It has found wide applications in chemical and biosensing fields owing to its high sensitivity, robustness, small sized-design, and ease of integration with electronic measurement systems.
chemical sensor
biosensor
Quartz Crystal Microbalance
1. Introduction
For more than 40 years, the Quartz Crystal Microbalance (QCM) has been one of the choices amongst many acoustic sensors due to its stability and sensitivity. The first application of quartz crystal was not a sensor but a resonator, mainly used in communications and oscillator circuits. QCM is a gravimetric sensor. Since mass is the fundamental property of an analyte, it can be monitored using acoustic devices. Its capability to detect the mass makes the acoustic resonator a universal transducer. QCM is capable to detect very slight mass changes, around a nanogram in real-time only using a simple measurement setup. Although other acoustic devices such as a SAW (Surface Acoustic Wave) device or FBAR (Film Bulk Acoustic Resonator) can be used to detect mass, the high Q-factor characteristic of QCM enables the detection of a subtle frequency shift in comparison with those devices even if the device structure and measurement setup are simple. If the attached film is not rigid but viscous, its viscoelasticity property can also be deducted from the Q-factor change. However, some measurement technique is required to extract this parameter, unlike resonant frequency, which can be estimated by the QCM oscillating frequency (if an oscillator circuit was used). Figure 1 summarizes the measured parameters that are most frequently used for different types of applications.
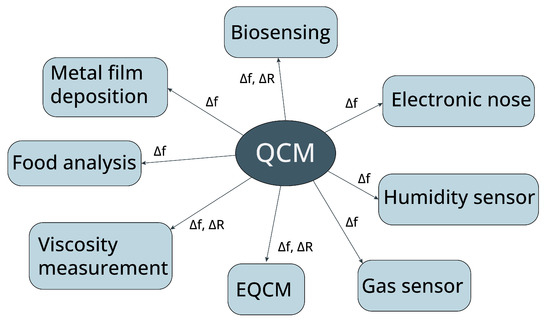
Figure 1. Measured parameters for QCM sensing applications. Δf is the QCM resonant frequency shift, and ΔR is the QCM equivalent resistance change.
QCM is a non-specific sensor and only detects the deposited mass changes. The traditional usage of a QCM sensor covers thickness monitors in metal evaporations and dissolution or corrosion [1]. Nowadays, the application expands into chemical and biosensing sensing both in the vapor phase and liquid phase. Sensing films are crucial for chemical and biosensing applications. Unfortunately, many coating materials with high selectivities and sensitivities are viscous, and viscous damping deteriorates the Q factor of QCMs.
Formerly, the QCM applications were based on an oscillator circuit where QCM is a resonator part of the circuit. Since the oscillation circuit ceases to operate due to high loss in acoustics loading, the use of viscous films or liquid-phase application becomes limited.
Nowadays, the advancements in oscillator circuit design benefit the QCM in terms of being able to operate even at a highly viscous loading [2][3]. Furthermore, some are even equipped with the function to extract the loss value or Q-factor from the QCM [4]. The classic approach to extract resonant frequency from QCM is the use of the Vector Network Analyzer (VNWA). Due to its large size and high cost, it is not a suitable measurement system for on-site applications. The next approach is to use a custom-designed circuit for QCM measurement. This approach was used by many researchers, and the commercial QCM measurement system is also based on the specially designed circuit. Lastly, the development of the portable-size VNWA has become ubiquitous. Many commercial products are available and have been adopted by many researchers.
QCM can also be used as a label-free sensor [5][6][7]. The concept of label-free is the recognition of the target analyte without tagging or labeling the analyte with enzymes or fluorescent or radioactive molecules. This is based on recognizing the analyte with the binding with materials. The detection is exclusively based on its intrinsic properties, thus reducing the cost and time of the labeling step. Trending on sensor research is focused on label-free detection protocols, and QCM is one of the suitable candidate transducers for this scheme.
2. Fundamental of QCM Sensor
2.1. The Piezoelectric Effect
The QCM sensor works based on the piezoelectric effect. A certain kind of crystal under strain becomes electrically polarized. When the materials deformed, the atoms were displaced. The displacement produces electrical dipoles in the material. If the summation of these dipoles generates moments, which occurs in noninversion symmetry material, this effect is called the direct piezoelectric effect. The effect can also be reversed by applying an electrical field to the crystal, and the internal mechanical strain is generated. This reverse piezoelectric effect is the working principle of QCM.
2.2. Physical Structure
The physical structure of QCM consists of a pair of electrodes coupled with piezoelectric crystal, and typically, quartz (SiO2) is used. If an alternating electric field with a frequency close to the resonant frequency of the crystal plate is applied to the electrode, the crystal vibrates intensely and stably (high Q factor). The simple structure of QCM is shown in Figure 2. The electrode acts an important role in energy trapping [8], which contributes to its very high Q factor, making it a very stable resonator for many electronic applications, the main oscillator, and timing circuits.
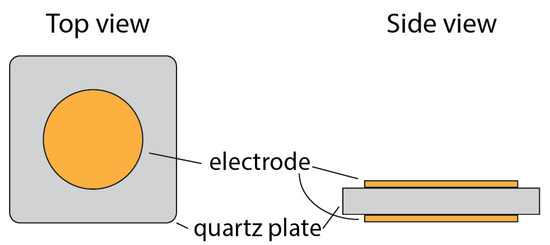
Figure 2. Basics structure of QCM.
2.3. Working Principle of QCM Sensor
QCM has its own natural frequency depending on the cutting angle and the thickness of the quartz crystal. The oscillation mode of QCM is called thickness-shear mode, which has the direction of displacement perpendicular to the quartz thickness with the maximum amplitude at the quartz plate surface, as shown in Figure 3.
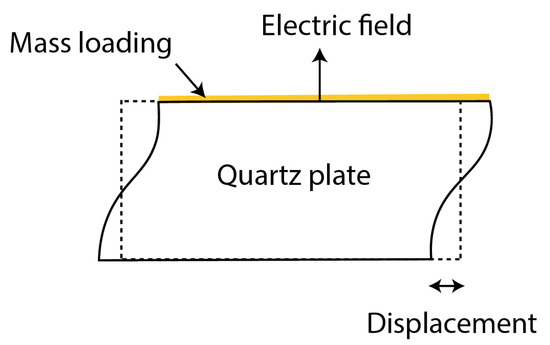
Figure 3. Thickness shear mode of Quartz Crystal Microbalance.
QCM detects the deposited mass and condition on the surface of the electrodes. For this thickness shear mode, the crystal thickness d is half of the shear mode wavelength, and we obtain
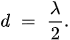
Let the velocity of the sound wave be v and its frequency f. Their relationship is
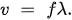
Therefore
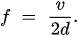
If the added mass acts as a thickness increase in the quartz per unit area increase by , then there will be a corresponding resonant frequency change as in Equation (4). This adding mass causes the reduction in resonant frequency.
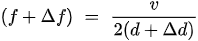
This is the simple explanation of the reason for the decrease in resonant frequency due to the film deposition. However, the frequency change is not governed by thickness change but mass change if the heterogeneous film is deposited.
It was found that the highest mass sensitivity of the QCM is obtained at the center, and it monotonously decreases at the location away from the center. The sensitivity curve can be approximated as a gaussian curve [9][10].
If the deposited layer over QCM is not rigid, one can expect the extra energy loss within that deposited material. This causes the Q-factor to decrease or the dissipation factor to increase. Conventional equations cover the cases of lossless film deposition and bulk liquid deposition but not the thin viscous film, which occurs in many applications.
3. Applications of QCM
The QCM has been greatly adopted as biosensors in recent years, such as the improvement of QCM sensitivity using liposome anchored on a double-stranded DNA [11] or with the imprinted polymer technology for insulin detection [12]. The detection of three strains of E. coli. was achieved by using antibody and the surface modification of QCM by Sulfo-SMCC [13]. They use both an oscillator circuit and vector network analyzer as measurement methods. The high fundamental frequency QCM (HFF-QCM) immunosensor with a frequency of 100 MHz was developed for the detection of pesticide carbaryl and thiabendazole [14]. The data were compared with standard and optimized ELISA (Enzyme-Linked Immuno Sorbent Assay), SPR (Surface Plasmon Resonance) and various low-frequency QCM sensors. They reported that 100 MHz HFF-QCM has higher sensitivity and better performance than SPR and QCMs with lower fundamental frequencies, and it achieved comparable performance as the standard ELISA method.
EQCM (Electrochemical Quartz Crystal Microbalance) is the technique that combines an electrochemical cell and a QCM sensor. This opens the possibility of detecting the mass deposition together with the ability to control and monitor the reactions and, at the electrode surface, using potentiostat. It was dated back as far as 1985 by Schumacher et al., who monitored the oxidation of a gold electrode and found that the surface roughness causes additional mass loading due to the trapped liquid [15]. Research in recent years also revolved around the study of the reaction of adsorption and deposition of battery mechanisms by using EIS (Electrochemical Impedance Spectroscopy) and EQCM to create a system for studying both physical and chemical changes [16]. This system employed the commercial QCM-D system, which can measure both resonant frequency and dissipation. This makes it possible to estimate film mass, film viscosity, film modulus and electrolyte viscosity from their model.
Furthermore, the applications of QCM in liquid viscosity measurements are still popular, such as a sensor that operates in harsh environments such as extreme temperature, acidity and pressure. B. Acharya et al. show the study of QCM in measuring high viscous oils at high temperatures [17]. Their apparatus consists of a stainless steel QCM chamber, and their temperatures range from 25 to 200 ∘
C. Their result shows good fitting with conventional equations with a correction factor based on the QCM surface roughness effect. The application of liquid viscosity measurement in a lead-acid battery was also reported [18]. The QCM sensor also has been used for detecting the flow assurance problem in the petroleum industry [19].Lastly, many commercially available measurement systems make it easier for researchers to use QCM without the need to create a measurement system. Table 1 is a selection of publications on QCM sensing from recent years.
Table 1. Selection of the recent QCM studies.
| Title | Application | Coating Material | Analyte | Features | Ref. |
|---|---|---|---|---|---|
| Fabrication of highly sensitive QCM sensor using anodic aluminum oxide (AAO) nanoholes and its application in biosensing | Biosensing | mouse IgG | anti-mouse IgG | Increased QCM sensor sensitivity with the AAO nano well structure on the measuring an antigen-antibody interaction | [20] |
| Acoustic methodology for selecting highly dissipative probes for ultrasensitive DNA detection | Biosensing | Liposome/ DNA complex | DNA | Liposomes anchored to a dsDNA chain led to an improvement of the limit of detection (LoD) by 3 orders of magnitude when compared to direct DNA detection | [11] |
| Target-triggering multiple-cycle signal amplification strategy for ultrasensitive detection of DNA based on QCM and SPR | Biosensing | Streptavidin-coated AuNPs (gold nanoparticles) | DNA | A signal amplification process, including the exonuclease III and the hybridization chain reaction (HCR) of DNA. The reaction was detected by a QCM sensor together with SPR sensor. It exhibited a high sensitivity toward target DNA with a detection limit of 0.70 fM | [21] |
| Classification of multiple Chinese Liquors by Means of a QCM-based E-Nose and MDS-SVM Classifier | Electronic nose | PVC, Polyamide, Polyethlyene, Polytef, AgCl, Azithromycin, CuCl2 | Various Chinese liquors | Identify the different types of Chinese liquors using an array of QCM with an SVM classifier. The prediction accuracy (98.3%) showed superior performance of the MDS-SVM classifier over the back-propagation artificial neural network (BP-ANN) classifier (93.3%) and moving average-linear discriminant analysis (MA-LDA) classifier (87.6%) | [22] |
| An investigation about the origin of the lung cancer signaling VOCs in breath | Electronic nose | RuTPP, RhTPP, MnTPP, CoTPP, SnTPP, CoNO2TPP, CoOCH3TPP, MnOMC | Exhale breath sample from cancer patients | Partial Least Squares Discriminant Analysis (PLS-DA) has been used. The electronic nose could discriminate between cancer and non-cancer patients with more than 90% correct classification | [23] |
| Electronic nose system based on Quartz Crystal Microbalance sensor for blood glucose and hba1c levels from exhaled breath odor | Electronic nose | Zeolites, fullerene C60, chiral materials, polypyrrole, carbon graphites, ITO films, oligonucleotides | Exhale breath sample | The study of exhale gas to detect Blood Glucose and HbA1c level using a radial basis function neural network (RBFNN). The accuracies were 83.03% and 74.76% for HbA1c parameter predictions and glucose parameter prediction | [24] |
| An ultrasensitive electrochemical impedance-based biosensor using insect odorant receptors to detect odorants | Electronic nose | Odorant receptors (ORs): Or10a, Or22a, Or35a, Or71a | Methyl salicylate, E2-hexenal, 4-ethylguaiacol | OrX/Orco liposomes could sensitively and selectively detect their ligands by monitoring a change in frequency and dissipation signal of Quartz Crystal Microbalance | [25] |
| Application of Quartz Crystal Microbalance with dissipation (QCM-D) to study low-temperature adsorption and fouling of milk fractions on stainless steel | Food analysis | Stainless steel(SS2343) | Whole milk, skim milk, acid whey, acid permeate | acid whey (pH 4.6) demonstrated significant constant-rate adsorption at long processing times. It is anticipated that linear adsorption rates at extended times can be used to predict fouling propensity at a commercial scale | [26] |
| Novel Quartz Crystal Microbalance immunodetection of aflatoxin B1 coupling cargo-encapsulated liposome with indicator-triggered displacement assay | Food analysis | Glucose-loaded nanoliposome, labeled with monoclonal anti-AFB1 antibody | Aflatoxin B1 | QCM response showed a good linear relationship between the frequency shift, and AFB1 concentration could be obtained within the dynamic working range from 1.0 ng kg−1 to 10 mg kg−1 | [27] |
| Fabrication of a Quartz Crystal Microbalance sensor based on graphene oxide/TiO2 composite for the detection of chemical vapors at room temperature | Gas sensing | Graphene oxide (GO)/TiO2 | Ethanol vapor | The sensitivity of the composite functionalized QCM resonator for the EtOH vapor ranged from 8300 to 20 ppm | [28] |
| Highly sensitive and chemically stable NH3 sensors based on an organic acid-sensitized cross-linked hydrogel for exhaled breath analysis | Gas sensing | Acid-sensitized cross-linked poly(ethylene glycol) diacrylate (PEGDA) hydrogel | NH3 | CA (Citric Acid)/PEGDA and MA(Malic Acid)/PEGDA sensors exhibit a response as low as 0.05 ppm NH3 | [29] |
| Humidity Sensing Properties of Metal Organic Framework-Derived Hollow Ball-Like TiO2 Coated QCM Sensor | Humidity sensor | TiO2 Nanopowder | Humidity | The sensor indicates a large frequency change with an interaction that occurred between TiO2 and humidity molecules. The sensor exhibited a good repeatability when it was exposed to the moist air of 65% RH | [30] |
| Quartz crystal microbalance apparatus for the study of viscous liquids at high temperatures | Viscosity measurement | N/A | Liquid viscosity | The study of QCM in measuring high viscous oils at high-temperature range from 25 to 200 ∘C | [17] |
| Resolution in QCM Sensors for the Viscosity and Density of Liquids: Application to Lead Acid Batteries | Viscosity measurement | N/A | Liquid viscosity | The application of liquid viscosity measurement in lead-acid battery. The findings show that the resolution limit only depends on the characteristics of the liquid to be studied and not on frequency | [18] |
| Operando EQCM-D with Simultaneous in situ EIS: New Insights into Interphase Formation in Li-Ion Batteries | EQCM | Super C65 Carbon, lithium iron phosphate (LiFePO4) | N/A | QCM with dissipation monitoring (EQCM-D) with simultaneous in situ electrochemical impedance spectroscopy (EIS) has been developed and applied to study the solid electrolyte interphase (SEI) formation on copper current collectors in Li-ion batteries | [16] |
| QCM sensing of bisphenol A using molecularly imprinted hydrogel/conducting polymer matrix | EQCM | Cyclodextrin-modified poly(L-lysine) (CD-PLL) | Bisphenol A (BPA) | The BPA-imprinted CD-PLL gel layer chip showed a greater Δf in response to BPA than the non-imprinted CD-PLL gel layer chip and the directly CD-immobilized chip | [31] |
References
- Oltra, R.; Efimov, I.O. Local sensitivity of an electrochemical quartz crystal microbalance: Spatial localization of the low frequency mode. Rev. Sci. Instrum. 1995, 66, 1136–1141.
- Avramov, I.D. A 0-phase circuit for QCM-based measurements in highly viscous liquid environments. IEEE Sens. J. 2005, 5, 425–432.
- Borngräber, R.; Schröder, J.; Lucklum, R.; Hauptmann, P. Is an oscillator-based measurement adequate in a liquid environment? IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002, 49, 1254–1259.
- Nakamoto, T.; Kobayashi, T. Development of Circuit for Measuring Both Q Variation and Resonant Frequency Shift of Quartz Crystal Microbalance. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1994, 41, 806–811.
- Noi, K.; Iwata, A.; Kato, F.; Ogi, H. Ultrahigh-frequency, wireless mems qcm biosensor for direct, label-free detection of biomarkers in a large amount of contaminants. Anal. Chem. 2019, 91, 9398–9402.
- Kirkendall, C.R.; Kwon, J.W. Sub-picogram resolution mass sensing in a liquid environment using low-loss quartz crystal microbalance. In Proceedings of the IEEE Sensors, Waikoloa, HI, USA, 1–4 November 2010.
- Huang, G.S.; Wang, M.T.; Su, C.W.; Chen, Y.S.; Hong, M.Y. Picogram detection of metal ions by melanin-sensitized piezoelectric sensor. Biosens. Bioelectron. 2007, 23, 319–325.
- Shockley, W.; Curran, D.R.; Koneval, D.J. Trapped-Energy Modes in Quartz Filter Crystals. J. Acoust. Soc. Am. 1967, 41, 981–993.
- Ward, M.D.; Delawski, E.J. Radial Mass Sensitivity of the Quartz Crystal Microbalance in Liquid Media. Anal. Chem. 1991, 63, 886–890.
- Huang, X.; Bai, Q.; Hu, J.; Hou, D. A practical model of quartz crystal microbalance in actual applications. Sensors 2017, 17, 1785.
- Milioni, D.; Mateos-Gil, P.; Papadakis, G.; Tsortos, A.; Sarlidou, O.; Gizeli, E. Acoustic Methodology for Selecting Highly Dissipative Probes for Ultrasensitive DNA Detection. Anal. Chem. 2020, 92, 8186–8193.
- Kartal, F.; Çimen, D.; Bereli, N.; Denizli, A. Molecularly imprinted polymer based quartz crystal microbalance sensor for the clinical detection of insulin. Mater. Sci. Eng. C 2019, 97, 730–737.
- Farka, Z.; Kovář, D.; Skládal, P. Rapid detection of microorganisms based on active and passive modes of QCM. Sensors 2015, 15, 79–92.
- March, C.; García, J.V.; Sánchez, Á.; Arnau, A.; Jiménez, Y.; García, P.; Manclús, J.J.; Montoya, Á. High-frequency phase shift measurement greatly enhances the sensitivity of QCM immunosensors. Biosens. Bioelectron. 2015, 65, 1–8.
- Schumacher, R.; Borges, G.; Kanazawa, K.K. The quartz microbalance: A sensitive tool to probe surface reconstructions on gold electrodes in liquid. Surf. Sci. 1985, 163, L621–L626.
- Kitz, P.G.; Lacey, M.J.; Novák, P.; Berg, E.J. Operando EQCM-D with Simultaneous in Situ EIS: New Insights into Interphase Formation in Li Ion Batteries. Anal. Chem. 2019, 91, 2296–2303.
- Acharya, B.; Sidheswaran, M.A.; Yungk, R.; Krim, J. Quartz crystal microbalance apparatus for study of viscous liquids at high temperatures. Rev. Sci. Instrum. 2017, 88, 025112.
- Cao-Paz, A.M.; Rodríguez-Pardo, L.; Fariña, J.; Marcos-Acevedo, J. Resolution in QCM sensors for the viscosity and density of liquids: Application to lead acid batteries. Sensors 2012, 12, 10604–10620.
- Abudu, A.; Goual, L. Adsorption of crude oil on surfaces using quartz crystal microbalance with dissipation (QCM-D) under flow conditions. Energy Fuels 2009, 23, 1237–1248.
- Asai, N.; Shimizu, T.; Shingubara, S.; Ito, T. Fabrication of highly sensitive QCM sensor using AAO nanoholes and its application in biosensing. Sens. Actuators B Chem. 2018, 276, 534–539.
- Song, W.; Guo, X.; Sun, W.; Yin, W.; He, P.; Yang, X.; Zhang, X. Target-triggering multiple-cycle signal amplification strategy for ultrasensitive detection of DNA based on QCM and SPR. Anal. Biochem. 2018, 553, 57–61.
- Li, Q.; Gu, Y.; Jia, J. Classification of multiple chinese liquors by means of a QCM-based e-nose and MDS-SVM classifier. Sensors 2017, 17, 272.
- Capuano, R.; Martinelli, E.; Ghezzi, S.; Paolesse, R.; Di Natale, C.; D’Amico, A.; Santonico, M.; Pennazza, G. An investigation about the origin of the lung cancer signalling VOCs in breath. In Proceedings of the IEEE Sensors, Valencia, Spain, 2–5 November 2014; Volume 2014.
- Saraoglu, H.M.; Selvi, A.O.; Ebeoglu, M.A.; Tasaltin, C. Electronic nose system based on quartz crystal microbalance sensor for blood glucose and hba1c levels from exhaled breath odor. IEEE Sens. J. 2013, 13, 4229–4235.
- Khadka, R.; Aydemir, N.; Carraher, C.; Hamiaux, C.; Colbert, D.; Cheema, J.; Malmström, J.; Kralicek, A.; Travas-Sejdic, J. An ultrasensitive electrochemical impedance-based biosensor using insect odorant receptors to detect odorants. Biosens. Bioelectron. 2019, 126, 207–213.
- Huellemeier, H.A.; Eren, N.M.; Ortega-Anaya, J.; Jimenez-Flores, R.; Heldman, D.R. Application of quartz crystal microbalance with dissipation (QCM-D) to study low-temperature adsorption and fouling of milk fractions on stainless steel. Chem. Eng. Sci. 2022, 247, 117004.
- Tang, Y.; Tang, D.; Zhang, J.; Tang, D. Novel quartz crystal microbalance immunodetection of aflatoxin B1 coupling cargo-encapsulated liposome with indicator-triggered displacement assay. Anal. Chim. Acta 2018, 1031, 161–168.
- Jayawardena, S.; Siriwardena, H.D.; Rajapakse, R.M.; Kubono, A.; Shimomura, M. Fabrication of a quartz crystal microbalance sensor based on graphene oxide/TiO2 composite for the detection of chemical vapors at room temperature. Appl. Surf. Sci. 2019, 493, 250–260.
- Liu, L.; Fei, T.; Guan, X.; Zhao, H.; Zhang, T. Highly sensitive and chemically stable NH3 sensors based on an organic acid-sensitized cross-linked hydrogel for exhaled breath analysis. Biosens. Bioelectron. 2021, 191, 113459.
- Zhang, D.; Chen, H.; Li, P.; Wang, D.; Yang, Z. Humidity Sensing Properties of Metal Organic Framework-Derived Hollow Ball-Like TiO2 Coated QCM Sensor. IEEE Sens. J. 2019, 19, 2909–2915.
- Matsumoto, K.; Tiu, B.D.B.; Kawamura, A.; Advincula, R.C.; Miyata, T. QCM sensing of bisphenol A using molecularly imprinted hydrogel/conducting polymer matrix. Polym. J. 2016, 48, 525–532.
More
Information
Subjects:
Engineering, Biomedical
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.4K
Revisions:
2 times
(View History)
Update Date:
18 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




