Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Biomedical
Quartz Crystal Microbalance (QCM) is one of the many acoustic transducers. It is the most popular and widely used acoustic transducer for sensor applications. It has found wide applications in chemical and biosensing fields owing to its high sensitivity, robustness, small sized-design, and ease of integration with electronic measurement systems.
- chemical sensor
- biosensor
- Quartz Crystal Microbalance
1. Introduction
For more than 40 years, the Quartz Crystal Microbalance (QCM) has been one of the choices amongst many acoustic sensors due to its stability and sensitivity. The first application of quartz crystal was not a sensor but a resonator, mainly used in communications and oscillator circuits. QCM is a gravimetric sensor. Since mass is the fundamental property of an analyte, it can be monitored using acoustic devices. Its capability to detect the mass makes the acoustic resonator a universal transducer. QCM is capable to detect very slight mass changes, around a nanogram in real-time only using a simple measurement setup. Although other acoustic devices such as a SAW (Surface Acoustic Wave) device or FBAR (Film Bulk Acoustic Resonator) can be used to detect mass, the high Q-factor characteristic of QCM enables the detection of a subtle frequency shift in comparison with those devices even if the device structure and measurement setup are simple. If the attached film is not rigid but viscous, its viscoelasticity property can also be deducted from the Q-factor change. However, some measurement technique is required to extract this parameter, unlike resonant frequency, which can be estimated by the QCM oscillating frequency (if an oscillator circuit was used). Figure 1 summarizes the measured parameters that are most frequently used for different types of applications.
QCM is a non-specific sensor and only detects the deposited mass changes. The traditional usage of a QCM sensor covers thickness monitors in metal evaporations and dissolution or corrosion [1]. Nowadays, the application expands into chemical and biosensing sensing both in the vapor phase and liquid phase. Sensing films are crucial for chemical and biosensing applications. Unfortunately, many coating materials with high selectivities and sensitivities are viscous, and viscous damping deteriorates the Q factor of QCMs.
Formerly, the QCM applications were based on an oscillator circuit where QCM is a resonator part of the circuit. Since the oscillation circuit ceases to operate due to high loss in acoustics loading, the use of viscous films or liquid-phase application becomes limited.
Nowadays, the advancements in oscillator circuit design benefit the QCM in terms of being able to operate even at a highly viscous loading [2,3]. Furthermore, some are even equipped with the function to extract the loss value or Q-factor from the QCM [4]. The classic approach to extract resonant frequency from QCM is the use of the Vector Network Analyzer (VNWA). Due to its large size and high cost, it is not a suitable measurement system for on-site applications. The next approach is to use a custom-designed circuit for QCM measurement. This approach was used by many researchers, and the commercial QCM measurement system is also based on the specially designed circuit. Lastly, the development of the portable-size VNWA has become ubiquitous. Many commercial products are available and have been adopted by many researchers.
QCM can also be used as a label-free sensor [10,11,12]. The concept of label-free is the recognition of the target analyte without tagging or labeling the analyte with enzymes or fluorescent or radioactive molecules. This is based on recognizing the analyte with the binding with materials. The detection is exclusively based on its intrinsic properties, thus reducing the cost and time of the labeling step. Trending on sensor research is focused on label-free detection protocols, and QCM is one of the suitable candidate transducers for this scheme.
2. Fundamental of QCM Sensor
2.1. The Piezoelectric Effect
The QCM sensor works based on the piezoelectric effect. A certain kind of crystal under strain becomes electrically polarized. When the materials deformed, the atoms were displaced. The displacement produces electrical dipoles in the material. If the summation of these dipoles generates moments, which occurs in noninversion symmetry material, this effect is called the direct piezoelectric effect. The effect can also be reversed by applying an electrical field to the crystal, and the internal mechanical strain is generated. This reverse piezoelectric effect is the working principle of QCM.
2.2. Physical Structure
The physical structure of QCM consists of a pair of electrodes coupled with piezoelectric crystal, and typically, quartz (SiO2) is used. If an alternating electric field with a frequency close to the resonant frequency of the crystal plate is applied to the electrode, the crystal vibrates intensely and stably (high Q factor). The simple structure of QCM is shown in Figure 2. The electrode acts an important role in energy trapping [13], which contributes to its very high Q factor, making it a very stable resonator for many electronic applications, the main oscillator, and timing circuits.
2.3. Working Principle of QCM Sensor
QCM has its own natural frequency depending on the cutting angle and the thickness of the quartz crystal. The oscillation mode of QCM is called thickness-shear mode, which has the direction of displacement perpendicular to the quartz thickness with the maximum amplitude at the quartz plate surface, as shown in Figure 3.
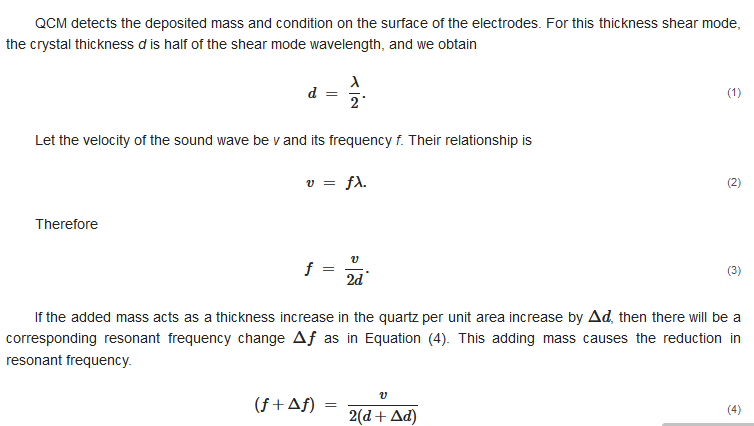
This is the simple explanation of the reason for the decrease in resonant frequency due to the film deposition. However, the frequency change is not governed by thickness change but mass change if the heterogeneous film is deposited.
It was found that the highest mass sensitivity of the QCM is obtained at the center, and it monotonously decreases at the location away from the center. The sensitivity curve can be approximated as a gaussian curve [18,19].
If the deposited layer over QCM is not rigid, one can expect the extra energy loss within that deposited material. This causes the Q-factor to decrease or the dissipation factor to increase. Conventional equations cover the cases of lossless film deposition and bulk liquid deposition but not the thin viscous film, which occurs in many applications.
3. Applications of QCM
The QCM has been greatly adopted as biosensors in recent years, such as the improvement of QCM sensitivity using liposome anchored on a double-stranded DNA [20] or with the imprinted polymer technology for insulin detection [21]. The detection of three strains of E. coli. was achieved by using antibody and the surface modification of QCM by Sulfo-SMCC [22]. They use both an oscillator circuit and vector network analyzer as measurement methods. The high fundamental frequency QCM (HFF-QCM) immunosensor with a frequency of 100 MHz was developed for the detection of pesticide carbaryl and thiabendazole [23]. The data were compared with standard and optimized ELISA (Enzyme-Linked Immuno Sorbent Assay), SPR (Surface Plasmon Resonance) and various low-frequency QCM sensors. They reported that 100 MHz HFF-QCM has higher sensitivity and better performance than SPR and QCMs with lower fundamental frequencies, and it achieved comparable performance as the standard ELISA method.
EQCM (Electrochemical Quartz Crystal Microbalance) is the technique that combines an electrochemical cell and a QCM sensor. This opens the possibility of detecting the mass deposition together with the ability to control and monitor the reactions and, at the electrode surface, using potentiostat. It was dated back as far as 1985 by Schumacher et al., who monitored the oxidation of a gold electrode and found that the surface roughness causes additional mass loading due to the trapped liquid [24]. Research in recent years also revolved around the study of the reaction of adsorption and deposition of battery mechanisms by using EIS (Electrochemical Impedance Spectroscopy) and EQCM to create a system for studying both physical and chemical changes [25]. This system employed the commercial QCM-D system, which can measure both resonant frequency and dissipation. This makes it possible to estimate film mass, film viscosity, film modulus and electrolyte viscosity from their model.
Furthermore, the applications of QCM in liquid viscosity measurements are still popular, such as a sensor that operates in harsh environments such as extreme temperature, acidity and pressure. B. Acharya et al. show the study of QCM in measuring high viscous oils at high temperatures [26]. Their apparatus consists of a stainless steel QCM chamber, and their temperatures range from 25 to 200 ∘
C. Their result shows good fitting with conventional equations with a correction factor based on the QCM surface roughness effect. The application of liquid viscosity measurement in a lead-acid battery was also reported [27]. The QCM sensor also has been used for detecting the flow assurance problem in the petroleum industry [28].Lastly, many commercially available measurement systems make it easier for researchers to use QCM without the need to create a measurement system. Table 1 is a selection of publications on QCM sensing from recent years.
This entry is adapted from the peer-reviewed paper 10.3390/chemosensors9120350
This entry is offline, you can click here to edit this entry!
