You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sofia Barbosa-Gouveia | + 1126 word(s) | 1126 | 2021-12-23 03:41:25 | | | |
| 2 | Rita Xu | Meta information modification | 1126 | 2022-01-13 03:34:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Barbosa-Gouveia, S. Novel Splicing Variant in Acylglycerol Kinase. Encyclopedia. Available online: https://encyclopedia.pub/entry/18143 (accessed on 22 December 2025).
Barbosa-Gouveia S. Novel Splicing Variant in Acylglycerol Kinase. Encyclopedia. Available at: https://encyclopedia.pub/entry/18143. Accessed December 22, 2025.
Barbosa-Gouveia, Sofia. "Novel Splicing Variant in Acylglycerol Kinase" Encyclopedia, https://encyclopedia.pub/entry/18143 (accessed December 22, 2025).
Barbosa-Gouveia, S. (2022, January 12). Novel Splicing Variant in Acylglycerol Kinase. In Encyclopedia. https://encyclopedia.pub/entry/18143
Barbosa-Gouveia, Sofia. "Novel Splicing Variant in Acylglycerol Kinase." Encyclopedia. Web. 12 January, 2022.
Copy Citation
Mitochondrial functional integrity depends on protein and lipid homeostasis in the mitochondrial membranes and disturbances in their accumulation can cause disease. AGK, a mitochondrial acylglycerol kinase, is not only involved in lipid signaling but is also a component of the TIM22 complex in the inner mitochondrial membrane, which mediates the import of a subset of membrane proteins.
acylglycerol kinase
mitochondrial dysfunction
mitochondrial ATP generation
1. Introduction
Mitochondria are responsible for several vital functions in mammalian cells, including adenosine triphosphate (ATP) production, steroid and stress hormones synthesis, cellular metabolism, and the induction of apoptosis [1][2][3]. Moreover, spatially and functionally regulated specific microdomains known as the mitochondria-associated membranes (MAMs) between the endoplasmic reticulum (ER) and mitochondria are hot spots of Ca2+ transfer, highlighting the mitochondria vital role in cellular Ca2+ homeostasis [4].
Mitochondrial diseases can arise due to defects in nuclear or mitochondrial DNA (nDNA and mtDNA, respectively) genes that encode proteins required for normal mitochondrial structure and/or function [5]. Acylglycerol kinase (AGK) is a mitochondrial membrane protein involved not only in lipid and glycerolipid metabolism but also in mitochondrial protein transport, glycolysis, and thrombocytopoiesis [6][7][8]. AGK can act as a lipid kinase to catalyze the phosphorylation of diacylglycerol (DAG) and monoacylglycerol (MAG) to phosphatidic acid (PA) and lysophosphatidic acid (LPA), respectively. Both PA and LPA can act as signaling molecules and participate in phospholipid synthesis [9][10], which is essential to maintain mitochondrial structure and function. Moreover, AGK scavenges mitochondrial DAG, which induces reactive oxygen species (ROS) signaling via a pathway mediated by protein kinase D1 [9][11].
Independently of its lipid kinase activity and its role in lipid metabolism stability, AGK acts as a subunit of the mitochondrial translocase of the inner membrane 22 (TIM22) complex [12][13], which is responsible for the translocation of transmembrane proteins from the cytoplasm into the mitochondrial interior [14] and their insertion into the mitochondrial inner membrane via the formation of a twin-pore translocase that harnesses membrane potential as the external driving force [14][15]. The TIM22 complex, which is required to import a subset of metabolite carriers into mitochondria, including ANT1/SLC25A4 and SLC25A24, consists of multiple subunits: Tim22, the core pore-forming subunit; the intermembrane space chaperones Tim9, Tim10, and Tim10b; and AGK and Tim29, which are specific subunits of the complex [14].
Sengers syndrome (OMIM #212350) is a rare autosomal recessive disorder caused by mutations in the AGK gene and characterized by hypertrophic cardiomyopathy, congenital cataracts, and mitochondrial myopathy, including muscle weakness and lactic acidosis after exercise [16]. Two clinical forms have been identified: a severe neonatal form that can cause infantile death due to heart failure consequent to hypertrophic cardiomyopathy, and a benign form with a better prognosis in which surviving patients achieve normal developmental milestones [9][17]. The pathological mechanism underlying Sengers syndrome remains unclear, although it is thought to involve AGK’s role in lipid metabolism.
2. Molecular Genetics and In Silico Analysis of the AGK Variant
NGS analysis enabled the identification of a homozygous variant, c.518+1G>A, in the AGK gene (NM_018238.4) in this patient. This splicing variant is located in intron 8, and segregation studies performed by Sanger sequencing confirmed that it was inherited from both parents (Figure 1A). The AGK variant was analyzed in silico to determine the degree of evolutionary conservation, predicted pathogenicity, functional consequences, and minor allele frequency (MAF) within the population. In addition, the Human Splicing Finder (HSF) [18] predicted that the splicing variant c.518+1G>A, located in intron 8, disrupts the wild-type splice donor site, likely affecting splicing (Figure 1B). Variants in the canonical acceptor and donor sites have been previously reported to strongly affect conserved sequences [19]. The 5′ splice site (donor site) and 3′ splice site (acceptor site) sequences are recognized by the elements of the spliceosome. Consequently, any variant located in these canonical sequences can alter the interactions between pre-mRNA and proteins involved in intron removal. Variants at the canonical splice sequences usually lead to single exon skipping [20]. The AGK gene encodes a 422-amino acid protein. Sequencing of our patient’s cDNA revealed a decrease in sequence length of 96 bp compared with control cDNA (Figure 1C), an effect likely due to exon 9 (96 bp) skipping during the splicing process.
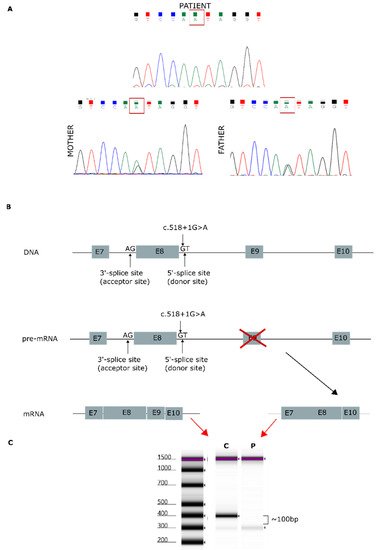
Figure 1. Genetic analysis findings. (A) Genomic DNA sequence chromatograms showing Sanger sequencing results. Both parents carried the splicing variant c.518+1G>A, located in intron 8. (B) AGK gene: predicted effect of exon 9 skipping during mature mRNA splicing. (C) cDNA sequencing of patient [P] and control [C], showing a 96-bp decrease in the length of the AGK cDNA sequence.
3. AGK Protein Modeling
The AGK protein consists of a typical two-domain fold (DGK domain 1 and DGK domain 2) that mediates phosphorylation of monoacylglycerols or diacylglycerols and has an N-terminal α1 helix that anchors to the membrane and a C-terminal key region with an additional membrane anchor helix loop (Figure 2) [6][21].
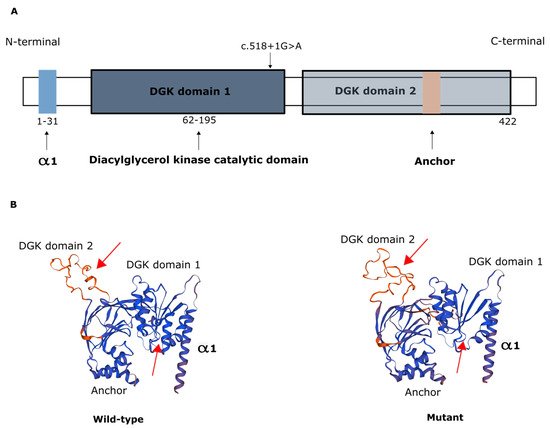
Figure 2. Representation of AGK protein domains. (A) Domain structure of AGK, which contains a two-domain fold (DGK domains 1 and 2) that mediates phosphorylation of monoacylglycerols or diacylglycerols, an N-terminal α1 helix (light blue) that anchors to the membrane, and a C-terminal region with an additional membrane anchor (light brown) helix loop. Schematic depicts the c.518+1G>A variant in the catalytic domain. (B) Protein modeling of wild-type AGK and of the AGK variant c.518+1G>A, showing predicted changes in spatial protein structure (red arrows).
4. Mitochondrial Respiration and OXPHOS Activity
Measurement of the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) revealed decreases in both OCR and the OCR:ECAR ratio in the patient’s fibroblasts, indicating reduced electron flow through the respiratory chain (Figure 3A,B). These findings reflect a general deficiency in the overall function of all mitochondrial respiratory chain complexes in the patient’s fibroblasts.
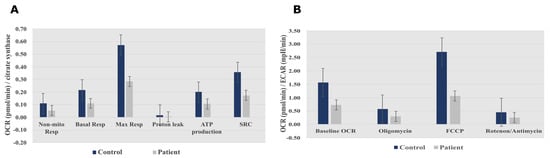
Figure 3. Seahorse XF96 respirometry analysis. (A) Oxygen consumption rate measured before and after the addition of inhibitors. These modulators are electron transport chain inhibitors (oligomycin, FCCP, and a mixture of rotenone and antimycin A), which are serially injected to measure ATP production, Max Resp, Non-Mito Resp, proton leak, SRC, and Basal Resp. (B) The basal energy metabolism of each cell line was assessed by determining OCR:ECAR ratios following sequential injection of the inhibitors. Abbreviations: Basal Resp, basal respiration; ECAR, extracellular acidification rate; FCCP, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone; Max Resp, maximal respiration; Non-Mito Resp, non-mitochondrial respiration; OCR, oxygen consumption rate; SRC, spare respiratory capacity. All data were obtained from two independent experiments, expressed as mean value ± deviation standard.
Spectrophotometric analysis of respiratory chain enzyme activity revealed deficiencies in complexes I and V in the patient’s fibroblasts (Table 1).
Table 1. Measurement of enzyme activities for the different OXPHOS complexes in patient and control fibroblasts (reference range determined for healthy population).
| Patient-Activity (mU/U CS) | Control-Activity (mU/U CS) | Reference Range | |
|---|---|---|---|
| Complex I | 142 | 320 | 163–599 |
| Complex II | 367 | 582 | 335–888 |
| Complex III | 592 | 628 | 570–1383 |
| Complex IV | 556 | 527 | 288–954 |
| Complex V | 103 | 712 | 193–819 |
Bold font indicates complex for which reduced activity was observed. Abbreviations: CS, citrate synthase.
References
- Anderson, A.J.; Jackson, T.D.; Stroud, D.A.; Stojanovski, D. Mitochondria—Hubs for regulating cellular biochemistry: Emerging concepts and networks. Open Biol. 2019, 9, 190126.
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial proteins: From biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 2019, 20, 267–284.
- Picard, M.; McEwen, B.S.; Epel, E.S.; Sandi, C. An energetic view of stress: Focus on mitochondria. Front. Neuroendocr. 2018, 49, 72–85.
- Kerkhofs, M.; Bittremieux, M.; Morciano, G.; Giorgi, C.; Pinton, P.; Parys, J.B.; Bultynck, G. Emerging molecular mechanisms in chemotherapy: Ca2+ signaling at the mitochondria-associated endoplasmic reticulum membranes. Cell Death Dis. 2018, 9, 1–15.
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial diseases. Nat. Rev. Dis. Primers 2016, 2, 16080.
- Chu, B.; Hong, Z.; Zheng, X. Acylglycerol Kinase-Targeted Therapies in Oncology. Front. Cell Dev. Biol. 2021, 9, 1948.
- Jiang, H.; Yu, Z.; Ding, N.; Yang, M.; Zhang, L.; Fan, X.; Zhou, Y.; Zou, Q.; Hou, J.; Zheng, J.; et al. The role of AGK in thrombocytopoiesis and possible therapeutic strategies. Blood 2020, 136, 119–129.
- Hu, Z.; Qu, G.; Yu, X.; Jiang, H.; Teng, X.-L.; Ding, L.; Hu, Q.; Guo, X.; Zhou, Y.; Wang, F.; et al. Acylglycerol Kinase Maintains Metabolic State and Immune Responses of CD8+ T Cells. Cell Metab. 2019, 30, 290–302.e5.
- Mayr, J.A.; Haack, T.B.; Graf, E.; Zimmermann, F.A.; Wieland, T.; Haberberger, B.; Superti-Furga, A.; Kirschner, J.; Steinmann, B.; Baumgartner, M.R.; et al. Lack of the Mitochondrial Protein Acylglycerol Kinase Causes Sengers Syndrome. Am. J. Hum. Genet. 2012, 90, 314–320.
- Bektas, M.; Payne, S.G.; Liu, H.; Goparaju, S.; Milstien, S.; Spiegel, S. A novel acylglycerol kinase that produces lysophosphatidic acid modulates cross talk with EGFR in prostate cancer cells. J. Cell Biol. 2005, 169, 801–811.
- Cowell, C.F.; Doppler, H.; Yan, I.K.; Hausser, A.; Umezawa, Y.; Storz, P. Mitochondrial diacylglycerol initiates protein-kinase-D1-mediated ROS signaling. J. Cell Sci. 2009, 122, 919–928.
- Vukotic, M.; Nolte, H.; König, T.; Saita, S.; Ananjew, M.; Krüger, M.; Tatsuta, T.; Langer, T. Acylglycerol Kinase Mutated in Sengers Syndrome Is a Subunit of the TIM22 Protein Translocase in Mitochondria. Mol. Cell 2017, 67, 471–483.e7.
- Kang, Y.; Stroud, D.A.; Baker, M.J.; De Souza, D.P.; Frazier, A.E.; Liem, M.; Tull, D.; Mathivanan, S.; McConville, M.J.; Thorburn, D.R.; et al. Sengers Syndrome-Associated Mitochondrial Acylglycerol Kinase Is a Subunit of the Human TIM22 Protein Import Complex. Mol. Cell 2017, 67, 457–470.e5.
- Jackson, T.D.; Hock, D.H.; Fujihara, K.M.; Palmer, C.S.; Frazier, A.E.; Low, Y.C.; Kang, Y.; Ang, C.-S.; Clemons, N.J.; Thorburn, D.R.; et al. The TIM22 complex mediates the import of sideroflexins and is required for efficient mitochondrial one-carbon metabolism. Mol. Biol. Cell 2021, 32, 475–491.
- Stojanovski, D.; Bohnert, M.; Pfanner, N.; Van Der Laan, M. Mechanisms of Protein Sorting in Mitochondria. Cold Spring Harb. Perspect. Biol. 2012, 4, a011320.
- Sengers, R.; Trijbels, J.; Willems, J.; Daniels, O.; Stadhouders, A. Congenital cataract and mitochondrial myopathy of skeletal and heart muscle associated with lactic acidosis after exercise. J. Pediatr. 1975, 86, 873–880.
- Haghighi, A.; Haack, T.B.; Atiq, M.; Mottaghi, H.; Haghighi-Kakhki, H.; Bashir, R.A.; Ahting, U.; Feichtinger, R.G.; Mayr, J.A.; Rötig, A.; et al. Sengers syndrome: Six novel AGK mutations in seven new families and review of the phenotypic and mutational spectrum of 29 patients. Orphanet J. Rare Dis. 2014, 9, 119.
- Desmet, F.-O.; Hamroun, D.; Lalande, M.; Collod-Beroud, G.; Claustres, M.; Béroud, C. Human Splicing Finder: An online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009, 37, e67.
- Krawczak, M.; Thomas, N.S.; Hundrieser, B.; Mort, M.; Wittig, M.; Hampe, J.; Cooper, D.N. Single base-pair substitutions in exon-intron junctions of human genes: Nature, distribution, and consequences for mRNA splicing. Hum. Mutat. 2007, 28, 150–158.
- Anna, A.; Monika, G. Splicing mutations in human genetic disorders: Examples, detection, and confirmation. J. Appl. Genet. 2018, 59, 253–268.
- Qi, L.; Wang, Q.; Guan, Z.; Wu, Y.; Shen, C.; Hong, S.; Cao, J.; Zhang, X.; Yan, C.; Yin, P. Cryo-EM structure of the human mitochondrial translocase TIM22 complex. Cell Res. 2021, 31, 369–372.
More
Information
Subjects:
Genetics & Heredity
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
755
Revisions:
2 times
(View History)
Update Date:
13 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




