Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Satyendra Chandra Tripathi | + 4938 word(s) | 4938 | 2021-12-28 11:14:37 | | | |
| 2 | Peter Tang | -5 word(s) | 4933 | 2022-01-13 07:53:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tripathi, S. Role of Immunoproteasome Subunits in Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/18141 (accessed on 08 February 2026).
Tripathi S. Role of Immunoproteasome Subunits in Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/18141. Accessed February 08, 2026.
Tripathi, Satyendra. "Role of Immunoproteasome Subunits in Cancer" Encyclopedia, https://encyclopedia.pub/entry/18141 (accessed February 08, 2026).
Tripathi, S. (2022, January 12). Role of Immunoproteasome Subunits in Cancer. In Encyclopedia. https://encyclopedia.pub/entry/18141
Tripathi, Satyendra. "Role of Immunoproteasome Subunits in Cancer." Encyclopedia. Web. 12 January, 2022.
Copy Citation
Cell-mediated immunity is driven by antigenic peptide presentation on major histocompatibility complex (MHC) molecules. Specialized proteasome complexes called immunoproteasomes process viral, bacterial, and tumor antigens for presentation on MHC class I molecules, which can induce CD8 T cells to mount effective immune responses. Immunoproteasomes are distinguished by three subunits that alter the catalytic activity of the proteasome and are inducible by inflammatory stimuli such as interferon-γ (IFN-γ). This inducible activity places them in central roles in cancer, autoimmunity, and inflammation.
ubiquitin–proteasome system (UPS)
immunoproteasome
solid tumors
proteasome inhibitors
1. Introduction
The ubiquitin-proteasome system (UPS) is a multicomponent, multiprotein structure that catalyzes the proteolysis of unwanted, misfolded, and foreign proteins that have been covalently modified with ubiquitin molecules [1]. Selective proteolysis performed by the UPS has been associated with almost every biological process within the cell [2]. The barrel-shaped 26S proteasome complex is composed of 20S core particles associated with two regulatory proteasome activator components. The core 20S complex is the catalytic site for protein degradation comprising multimeric subunits assembled in a ring structure [3]. Immunoproteasomes were first discovered in the early 1990s, with the observation that several proteasome subunits were induced by the pro-inflammatory cytokine IFN-γ [4][5]. Proteasomes produced with these inducible subunits carried a markedly altered catalytic activity, with increased levels of trypsin- and chymotrypsin-like activity and decreased levels of caspase-like activity [6].
The immunoproteasome carries out proteasomal degradation of protein substrates for the MHC class I restricted antigen processing pathway [7][8]. These endogenous antigenic peptides are then translocated across the ER by a transporter associated with antigen processing protein (TAP) for MHC presentation on the cell surface [9]. The MHC class I-peptide complexes are responsible for the activation of CD8+ T-cells through binding the T-cell receptor (TCR), activating the T-cell for mounting immune responses against intracellular pathogens [10]. The altered catalytic function of the immunoproteasome has been suggested to generate peptides suitable for presentation in the MHC cleft, producing peptides around 13-25 residues in length and often with hydrophobic C-termini [5][11][12]. Although the exact role of the altered catalytic function in generating these peptides is still under investigation, it is documented that a diverse range of antigenic peptides is produced through immunoproteasome activity, inducing CD8+ T-cell responses to a broad range of stimuli [13][14]. The difference in epitope generation between the constitutive and the immunoproteasome has been assigned to certain cleavage preferences for both proteasomes. This difference in substrate specificity may impact the immunopeptidome by altering the quantity of certain epitopes. This appears to be only partly explained by the increased preference of the immunoproteasome for specific P1 residues and cleavage following bulky hydrophobic amino acid residues [15]. Both proteasome isoforms also have a different production kinetics affecting quantity of epitopes [16]. Apart from its function in cell-mediated immunity, the immunoproteasome has been shown to have significant roles in inflammation, autoimmunity, and cancer. There has been an ever-growing list of novel functions of the immunoproteasome in regulating inflammatory processes, cytokine secretion, as well as facilitating protein homeostasis, cell differentiation, and cell signaling [17][18][19].
Due to its myriad functions, the immunoproteasome has become a focus in the investigation of the pathology of autoimmune conditions, cancer, inflammatory diseases, and neurodegenerative disorders (Figure 1). In tumorigenesis, there have been several reports regarding dysregulation of immunoproteasome expression and function [20]. Studies have found that tumors express immunoproteasome subunits in a dynamic fashion, which could be correlated to disease outcomes and survival [21][22][23][24][25][26][27]. Immunoproteasome inhibitors have been studied in clinical settings against solid tumors as well as hematological malignancies, but therapeutic targeting of the immunoproteasome in tumors has only shown modest success [28][29].
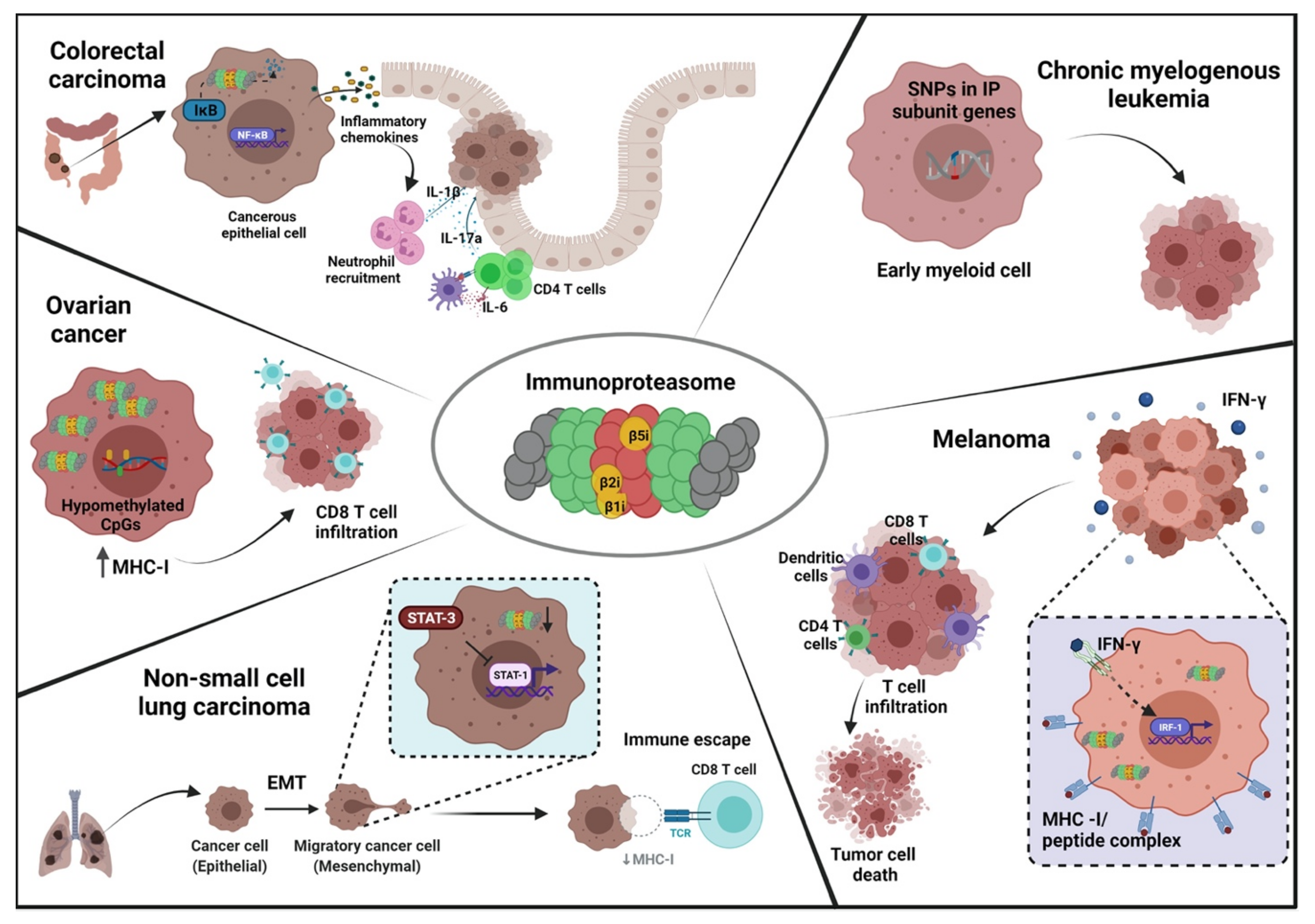
Figure 1. Schematic overview depicting various mechanisms of immunoproteasome participation in different cancers. Immunoproteasome can promote or inhibit tumorigenesis in various cancers through distinct and often contradictory mechanisms. In the colon, immunoproteasomal mediated degradation of IκB allows the generation of pro-inflammatory signals that eventually lead to neoplastic transformation of colonic epithelial cells. In melanoma, the inflammatory stimulus of IFN-γ increases the tumor antigen presentation and T cell infiltration, culminating in tumor cell death. In chronic myelogenous leukemia, the early myeloid cells have increased susceptibility to CML if they possess SNPs in the immunoproteasome subunit genes. In ovarian cancer cells, epigenetic modification of CpG islands promotes CD8 T cell migration into the tumor and induces CTL-mediated tumor killing. In non-small cell lung carcinoma, EMT is responsible for reducing immunoproteasome expression, thereby facilitating immune escape due to loss of MHC class I antigen presentation. EMT: epithelial to mesenchymal transition, IP: immunoproteasome, CTL: cytotoxic T lymphocytes.
2. Structural and Functional Differences: Constitutive and Immunoproteasome
2.1. Composition, Assembly, and Regulation
The prominent role of the UPS in immunity has emerged over the last three decades. The genes for several components of the UPS, including TAP genes and 20S subunits, were found to be located within the genomic regions containing MHC class-II genes [30][31][32]. Soon after, the proteasome was reported to have a crucial function in antigen processing for the MHC class-I presentation [4]. Studies revealed that IFN-γ induced changes in the levels and the composition of proteasomal subunits, producing a central core with altered catalytic activity. The resulting protein complex was named the immunoproteasome to highlight its role in the processing and presentation of endogenous antigens [5][11][12][33][34]. The immunoproteasome shares structural similarities in its scaffold with the constitutive proteasome, which has alternatively been called the 26S proteasome. Its supramolecular structure is a cylindrical protein complex composed of catalytic 20S core particle (CP) and two regulatory components covering the two ends of the barrel-shaped molecule. The 20S core particle consists of two pairs of heptameric ring structures. The two inner rings are built from seven β-subunits (β1-7), with the two outer rings consisting of seven α-subunits (α1-7) [3][35]. The catalytic properties of the CP in the constitutive proteasome are attributed to the β1, β2, and β5 subunits of the inner rings, with each subunit possessing distinct proteolytic activity [36].
In the generation of the immunoproteasome, β1, β2, and β5 are replaced by more efficient IFN-γ inducible subunits, which are termed β1i, β2i, and β5i. β1i is also known as large multifunctional peptidase 2 (LMP2) and is encoded by the gene Proteasome Subunit Beta type 9 (PSMB9). β2i is also known as LMP10 or multi-catalytic endopeptidase complex-like-1 (MECL-1) and is encoded by the PSMB10 gene. β5i is alternatively called LMP7 and is encoded by the PSMB8 gene [37][38]. The outer α-rings associate with the regulatory complexes that cap the two ends of the CP to allow the entry of substrates into the catalytic core, and thus serve as proteasome activators. Typically, three complexes termed PA28 (11S proteasome activator), PA200, and PA700 (19S proteasome activator), interact with the α-subunits [39]. Similar to inducible β-subunits, inflammatory stimuli like IFN-γ induce selective association with the PA28 complex and the 20S CP to form the immunoproteasome. The PA28 regulatory complex is a heptameric protein structure, composed of two homologous α (PSME1) and β (PSME2) subunits forming a heteroheptamer. A homoheptameric variant of PA28, composed of only of one γ subunit, (PSME3) typically occurs in the nucleus [40][41].
The basic assembly of the 20S core particle is similar for both the constitutive and immunoproteasome [42]. Synthesis begins with the formation of the outer heptameric α-rings, assisted by the proteasome-assembling chaperons (PAC proteins). The PAC1/2 chaperones stabilize the nascent outer ring complex, while PAC3/4 facilitates the formation of the ring structure by allowing the end subunits to join [43]. β-ring assembly begins with the recruitment of the β2 subunit by PAC3, with addition to the nascent α-ring structure. Next, PAC3/4 dissociates, allowing the remaining β-subunits, in a defined order of β3, β4, β5, β6, β1, and finally β7, to become incorporated into a half-formed proteasome with the outer α-ring [43][44]. Further assembly of the 20S core particle is mediated by chaperone proteasome maturation protein (POMP or proteassemblin), which fuses the two half-proteasomes. The final assembly of the 20S core requires cleavage of N-terminal pro-peptides on the catalytic β subunits to reveal a threonine residue in the active site [44]. In cells expressing both constitutive and inducible catalytic β-subunits, the assembly of the immunoproteasome is favored over the constitutive proteasome [45]. In contrast to standard proteasome assembly, the incorporation of β1i is essential for the addition of β2i subunit [42]. Next, the incorporation of β5i facilitates the maturation of the immunoproteasome by cleaving the pro-peptides from β1i and β2i [46][47]. Biogenesis of the immunoproteasome is also dependent on POMP, which is also transcriptionally induced by IFN-γ [48]. The selective preference for the synthesis of immunoproteasome over the constitutive proteasome has been attributed to the higher binding affinity of POMP to β5i over β5 [49][50].
Incorporation of the PA28 proteasome activator to the core particle to form the proteolytically active 26S immunoproteasome is an ATP-independent process [51]. The PA28 regulatory complex can associate with the CP as a single unit capping only one side, or as a pair covering both ends, or in combination with another regulator such as PA700 on either side. Cells expressing both constitutive and immunoproteasome subunit genes may also form a hybrid between the two, which have been termed intermediate proteasomes [52][53][54]. Generally, an intermediate proteasome will be composed of only one (β5i) subunit or two subunits (β5i and β1i) which are incorporated in place of the constitutive subunits [55]. The immunoproteasomes have a relatively shorter half-life due to their transient and inducible nature [48]. Similar to their constitutive counterparts, β2i and β5i subunits exhibit trypsin-like and chymotrypsin-like enzymatic activities respectively, with the similar peptide specificity. However, the β1i subunit displays more chymotrypsin-like activity as opposed to the caspase-like activity such as the β1 subunit [56][57].
The transcriptional regulation of the immunoproteasome is mediated by multiple pathways. The IFN-γ cytokine network is the most established inducer of immunoproteasome subunits, along with other antigen processing machinery such as TAP-1, PA28, and MHC class I and class II molecules [40][58][59]. Upon activation of IFN-γ signaling, the downstream mediators signal transducer and activator of transcription-1 (STAT-1) and IFN-γ regulatory factor 1 (IRF-1) upregulate the expression of catalytic βi subunits [58][60][61]. PA28αβ is upregulated via inhibition of PA700 and its preferential incorporation into the immunoproteasome through dephosphorylation of 20S core particle, both of which are also mediated by IFN-γ [62]. Type-I interferons IFN-α and IFN-β also regulate the immunoproteasome expression, which was demonstrated after hepatitis C and coxsackievirus infection [63][64][65]. Tumor necrosis factor-alpha (TNF-α) also has been shown to upregulate immunoproteasome expression upon liposaccharide mediated inflammatory stimulus [66]. Induction of the immunoproteasome has also been observed after nitric oxide (NO) exposure, constituting a cytokine-independent regulatory mechanism. This occurs through NO-mediated activation of c-AMP/PKA axis, leading to nuclear translocation of cAMP-responsive element-binding protein (CREB), which induces immunoproteasome subunit genes [67]. Apart from cellular mediators, environmental stressors can induce immunoproteasome, for instance exposure to heat shock and H2O2 [68][69]. Metabolic signals, including hyperglycemia has also been reported to regulate immunoproteasome expression [70]. Additionally, transcription factors such as NFκB, AP-1, Sp1 and Zif268 (also known as Egr1) also control transcription of individual immunoproteasome subunits [71][72][73].
3. Functional and Mechanistic Role of Immunoproteasome Subunits in Cancer
Neoplastic transformation is mediated by massive changes in cellular homeostasis. Induction of protein synthesis, a higher mutational burden, erroneous RNA splicing, and imbalanced redox environment due to metabolic changes all contribute to the production of misfolded or damaged proteins, requiring upregulation of protein turnover pathways [74][75]. Proteasome upregulation is a well-known contributor to tumorigenesis and was first described in breast cancer and multiple myeloma [29][76][77]. High proteasomal expression is necessary to overcome cellular stress pathways, and in some cases, to selectively degrade tumor suppressor proteins. The immunoproteasome has been shown to process tumor antigens and thereby influence both immune surveillance and immune escape (Figure 1) [78].
3.1. Role of Inducible Catalytic Subunits in Cancer
The generation of MHC class-I peptides is an important facet of the maturation of cytotoxic T cells (CTLs). Given the central function of CTLs in mounting anti-tumor responses, immunoproteasome subunits induced by IFN-γ have been studied for their assumed role in cancer development. Amongst the three IFN-γ inducible β subunits, β5i has to date been most implicated in blood and solid malignancies. This subunit, encoded by PSMB8 gene, has a wide range of expression among different cancers, which have been evaluated in non-small cell lung carcinoma (NSCLC), renal cell carcinoma, glioma, colorectal cancer, triple-negative breast carcinoma (TNBC), laryngeal, and hypopharyngeal carcinoma [21][22][79][80][81][82]. In many tumors, higher expression of PSMB8 has been linked with poor prognosis. PSMB8 expression was found to be upregulated in all histological sub-types of renal cell carcinoma [83]. Similarly, microarray profiling of gastric adenocarcinoma samples revealed that PSMB8 expression in tumor tissue was associated with poor prognosis [23]. High levels of PSMB8 are associated with more aggressive gliomas, and inhibition of PSMB8 was shown to reduce glioma cell proliferation and migration, as well to decrease glioblastoma tumor angiogenesis [79][84]. However, the observation that high expression of PSMB8 correlated with lower overall survival does not hold for all types of neoplasms. In NSCLC patients, high expression of PSMB8 was frequently observed in cancers with more favorable outcomes [22]. Likewise, increased PSMB8 expression in TNBC tumor samples was associated with better disease-free outcomes, including in those with metastatic disease [21].
The ambiguous role for PSMB8 in oncogenesis and disease progression seems to hinge on the fact that high levels of immunoproteasome expression can facilitate or impede tumor development in different contexts. For instance, the pro-tumorigenic role of PSMB8 in colorectal cancer is related to its role in colitis-induced chronic inflammation, which can drive neoplastic transformation of intestinal epithelium in the colon. Knockout of PSMB8 in mice was shown to prevent colitis-associated carcinogenesis [82]. PSMB8-deficient mice were found to be resistant to chronic inflammation and neoplasia, with reduced expression of chemokines CXCL-1, CXCL-2, and CXCL-3. Upon induction of colitis, PSMB8−/− mice did not show macroscopic tumor development. The authors further attributed the pro-tumor effects of PSMB8 to reduced secretion of IL-17A in inflamed colons of PSMB8 deficient mice. The study proposed that IL-17A secretion was PSMB8-dependent via the NFκB signaling axis. The immunoproteasome has been shown to directly regulate NFκB signaling via direct proteolytic degradation of IκB, with knockdown of PSMB8 preventing the nuclear translocation of NFκB [85][86]. In its role in inflammation-driven carcinogenesis, PSMB8 serves as a promising treatment target for colorectal carcinomas. Supporting this, a study showed that ONX-914, an immunoproteasome inhibitor with a higher affinity for β5i subunit, suppressed tumor development in both preventive and therapeutic settings of colitis-induced carcinogenesis [87].
However, as mentioned, deficiency of PSMB8 is context-dependent. PSMB8 deficiency has been shown to promote tumor growth in a mouse model of melanoma. It has been observed that PSMB8−/− mice implanted with B16 tumors have significant tumor growth and disease development [88]. In the absence of all three inducible subunits, mice failed to mount any anti-tumor immunity against the B16 melanoma cells, which was reflected in reduced CD8+ T cells in the draining lymph nodes and CTLs in the tumor microenvironment (TME) and decreased IFN-γ expression [88]. This study postulated that in melanoma carcinogenesis, IFN-γ induced immunoproteasome expression by tumor cells increases infiltration of immune cells, further adding to the pool of cytokine and chemical mediators in the TME and further upregulating IFN-γ secretion, which can exert its anti-tumor functions. IFN-γ mediated overexpression of LMP7 in melanoma cells might increase the generation of neo-antigenic peptides, further accentuating an anti-tumor response. In support of this, overexpression of PSMB8 in melanoma cell lines increased IFN-γ secretion, leading to efficient killing of tumor cells by tumor infiltrating CTLs. This seemed to be mediated through the presentation of more diverse and immunogenic HLA-1 peptides generated through overexpression of immunoproteasome subunits [89].
Thus, reduced expression of immunoproteasome subunits is a possible immune evasion mechanism deployed by tumor cells. In lung cancer, as in melanoma, higher expression of PSMB8 is associated with a more favorable prognosis, perhaps through increased immune surveillance [22][90]. In non-small cell lung carcinoma (NSCLC), tumor cells with lower expression of immunoproteasome subunits exhibited a more mesenchymal phenotype as opposed to the epithelial morphology of NSCLC cells with higher expression levels. Along with the mesenchymal phenotype, these tumor cells possessed increased migration and invasion ability with upregulated epithelial-to-mesenchymal transition (EMT) markers. Furthermore, STAT1 signaling was inhibited via the STAT3/mTOR regulatory axis in low PSMB8 expressing NSCLC cells. STAT1, in a mutually inhibitory relationship with STAT3, was shown to be a major downstream signaling molecule, controlling IFN-γ related genes including immunoproteasome and antigen presentation machinery. Upon treatment with IFN-γ, the mesenchymal phenotype of the tumor cells was reversed and phosphorylation of STAT1 was increased. Immunoproteasome induction in the mesenchymal-like NSCLC cell lines was shown to generate an increased diversity and quantity of MHC class I peptides. When pulsed with these generated peptides, autologous CD8 T cells demonstrated robust effector responses against tumor cells in vitro [90]. Thus, IFN-γ treatment induced immunoproteasome could potentially reverse this mechanism of tumoral immune evasion.
Immunoproteasomal subunit expression has also been reported to function as an indicator for treatment response and acquisition of chemoresistance. In both NSCLC and small cell lung cancer (SCLC), acquisition of cisplatin resistance correlated with increased expression of PSMB8 and PSMB9. Treatment of cisplatin-resistant tumor cells with proteasome inhibitors led to apoptosis induction, cell cycle arrest, and mitotic catastrophe. The authors propose that upregulation of immunoproteasome expression was a response to circumvent the cellular stress induced by cisplatin treatment [91]. Sensitivity to proteasome inhibitors by tumor cells was found to be associated with immunoproteasome subunit expression. In solid and hematological tumors, cells with low expression of immunoproteasome subunits showed poor response to proteasome inhibition, with significantly lower levels of apoptosis than cells with higher expression. However, pre-exposure with IFN-γ, which favored immunoproteasome subunit expression and immunoproteasome assembly, enhanced sensitivity to proteasome inhibitors [92]. As mentioned above, this raises a possibility of induction of the immunoproteasome through the IFN-γ pathway activation as a therapeutic strategy. For instance, resistance of the proteasome inhibitor bortezomib is associated with downregulation of PSMB8, which can be rescued through exogenous IFN-γ, leading to resensitization [93]. The resistance to inhibitor bortezomib was also found to be associated with mutation in the PSMB8 gene loci in multiple myeloma, which further potentiates the significance of screening PSMB8 mutations as well as expression for detecting chemoresistance to therapy [94].
A similar finding was noted in breast cancer. In TNBC, sensitivity to proteasome inhibitor treatment strongly correlated with high PSMB8 expression, with cells exhibiting UPS-driven apoptosis in response to immunoproteasome ablation [95]. To maintain high proliferative and invasive capacity, tumor cells increase protein turnover. Immunoproteasome upregulation by breast cancer cells is protective against increased proteotoxicity, which forms the part of unfolded protein response. Immunoproteasome upregulation, in this context, conceivably could be clinically targeted to overcome immunoproteasome driven chemoresistance, or could become a prognostic indicator of treatment responsiveness. Immunoproteasome expression was evaluated as a predictive marker for immune checkpoint blockade therapy in melanoma, with high expression of PSMB8 and PSMB9 associated with better response to anti-PD-1 and anti-CTLA-4 treatment [89].
PSMB8 expression in tumor cells not just reprograms the cellular pathways within the cell but also affects the tumor microenvironment. In highly invasive glioblastoma, a nexus of cellular communication is maintained between tumor cells, endothelial cells, and the extracellular matrix to allow increased angiogenesis. PSMB8 expression was reported to regulate this cellular communication. Elevated expression of PSMB8 was found in resected glioblastomas, and inhibition of PSMB8 reduced the migration and invasion of tumor cells in vitro. Endothelial cells demonstrated similar reduced migratory and tubulogenic properties when co-cultured with conditioned media taken from PSMB8-inhibited glioblastoma cell cultures. This interaction seemed to be mediated through reduced expression of vascular endothelial growth factor-A (VEGF-A) by tumor cells and integrin expression by endothelial cells. This was supported by a mouse model, which demonstrated that PSMB8 inhibition decreased tumor vessel formation [84]. However, the mechanism of VEGF-A control by the β5i subunit remains unexplained, with the authors hypothesizing immunoproteasome mediated degradation mechanism. Other studies have reported that PSMB8 regulation of migration and proliferation in less invasive grades of gliomas was dependent on PI3K and ERK pathways [80]. In addition to transcriptional and cytokine control of PSMB8 in cancer, regulation of PSMB8 has been reported through microRNAs, with miR-451a shown to target PSMB8 in prostate and thyroid cancer to prevent tumor cell proliferation and invasion [24][96].
The roles of the other two catalytic subunits, encoded by PSMB9 and PSMB10, are less described in cancer. β2i, or MECL1, encoded by PSMB10, has been reported to be downregulated in metastatic breast carcinoma, NSCLC, and acute promyelocytic leukemia however its functional relevance in tumor development is yet to be determined [90][97]. A recent study has implicated polymorphisms in PSMB10 as a genetic risk factor for chronic myelogenous leukemia (CML) [98]. The β1i subunit, encoded by PSMB9, was found to be reduced in breast cancer, renal cell carcinoma, APL, and NSCLC while elevated in melanoma and ovarian cancer [81][89][90][97][99][100][101][102]. Studies have reported a similar dichotomy as seen for PSMB8 regarding association with overall survival. Higher expression of PSMB9 in melanoma tumors has been linked with better patient outcomes while lower expression levels in NSCLC cells exhibited better prognosis [89][90]. A recent retrospective study on immune checkpoint therapy response for NSCLC and melanoma cohorts delineated a genetic signature of antigen processing and presentation (APM) genes which included PSMB9. Higher APM scores, and higher PSMB9 expression, correlated with better responses for immune checkpoint therapy (ICB) in both NSCLC and melanoma with improved overall survival [102].
The regulation of catalytic βi-subunits in cancer is brought about by several mechanisms. As described, NFκB, mTOR, and STAT1 have been shown to regulate the expression of PSMB8 in colon and lung cancer [87][90][91]. In acute promyelocytic leukemia (APL), the fusion transcription factor PML/RARα resulting from the causative chromosomal rearrangement (15;17) has been shown to interact with transcription factor PU.1 to repress the expression of all βi subunits [97]. As described above, contextual suppression of the immunoproteasome may provide a route for immune evasion, while upregulation may impart resistance to proteotoxicity. Aneuploidy, a common feature of neoplastic transformation, often increases protein production. Increased proteasomal degradation of tumor suppressor genes is another potential exacerbator of tumorigenesis. Constitutive proteasomal subunits are also frequently dysregulated during tumor initiation, and the induction of immunoproteasome subunits could provide extra capacity to cells undergoing intense protein turnover.
Apart from transcriptional control, immunoproteasome subunits are also regulated epigenetically. Hypomethylation of 6p21.3 CpG islands in high-grade serous epithelial ovarian carcinoma upregulates PSMB8/9 along with antigen presentation machinery proteins. This was found to be associated with increased time until recurrence time and increased CD8 T cell infiltration [100]. Low methylation profiles were observed for PSMB8 genomic regions in mucinous type epithelial ovarian cancers, which correlated with increased susceptibility to proteasome inhibitors [103]. Epigenetic modification of immunoproteasome subunits occurs diversely and is tumor-specific to which part of tumorigenesis it affects. Besides the regulation at the transcriptional and epigenetic level, immunoproteasome subunits themselves exhibit genetic polymorphisms which serve as susceptibility markers for certain cancers such as CML, cervical, and colon cancer [98][104][105].
3.2. Role of Regulatory Subunits in Cancer
Even though immunoproteasome can process varied kinds of protein substrates, association with PA28 plays an important role in the generation of CTL-specific epitopes, by alerting conformation of the α rings [106][107][108][109]. While PA28 is also inducible by IFN-γ, it is also induced upon LPS or CD40 stimulation in dendritic cells [110]. The α and β subunits of the PA28 complex are differentially expressed and regulated independently. Since studies have shown that this dynamic expression influences clinical outcomes in various cancers, it has spiked the interest as to whether the differential expression and IFN-γ independent regulation of PA28 could independently promote the generation of tumor neoantigens.
In ovarian cancer, the C-terminal fragment of PA28 (PA28S or Reg-alpha, encoded by the PSME1 gene) was found in tumor biopsies with its presence correlated with poorer overall survival in patients, and was designated as a reliable biomarker to monitor tumor relapses and treatment [111]. Similarly, in multiple myeloma, the patients with a higher abundance of PA28α in their plasma showed reduced response to the proteasome inhibitor bortezomib [26]. Just as with the IFN-γ inducible catalytic subunits, the role of regulatory subunit expression in tumors is context-dependent. It has been reported that in oral squamous cell carcinoma (OSCC) and soft tissue leiomyosarcoma, high expression of PA28α in tumor samples corresponds with poor prognosis, while in melanoma, elevated levels of PSME1 were associated with better overall survival [25][27][112]. In OSCC cells, inhibition of PA28α in vitro led to decreased cell proliferation and a significant reduction in invasion ability and migration, implying a role in tumor growth and metastasis [112]. A similar role was shown in breast cancer cell lines, where PA28 inhibition was shown to increase CDK15, leading to suppression of migration and invasion [113][114]. Conversely, expression of PA28β was downregulated in esophageal squamous cell carcinoma, with overexpression inhibiting tumor cell proliferation in vitro [115]. However, there are limited functional studies to understand the mechanism underlying the differential behavior of PA28 and its subunits in cancer development. A recent study highlighted individual pathways of regulation for each subunit in cutaneous melanoma. Gene set enrichment and pathway-based analysis of the individual PSME genes showed independent and often contrasting pathways, for instance, PSME1 expression was positively correlated with increases in cell adhesion, apoptosis, and NFκB and Wnt signaling pathways while PSME2 was negatively correlated with the same [25]. PSME3 seemed to share features of PSME1 regulation, with correlation to NFκB and Wnt signaling pathways.
Besides its role as a prognostic marker, the PA28 complex also has been studied for its feasibility as a predictive marker for treatment response. PSME1 and PSME2 were included as part of the APM score described above that described responsiveness to ICB in NSCLC and melanoma [102]. This finding, however, is not consistent across all immunotherapies. PA28 was found to prevent effective responses in antigen-specific immunotherapy against melanoma. The protein MART-1 (also known as Melan- A or melanoma antigen recognized by T cells) has been investigated as a potential target for immunotherapy but initial trials showed a poorer than expected immune response. In vitro studies showed that the immunodominant MART-1 epitope was not efficiently recognized by CD8 T cells, due to epitope destruction by unexpected cleavage mediated by the PA28 complex [115]. In ICB, expression of the entire PA28 was observed to be a positive response marker. Alternatively, expression of PSME1 was found to be indicative of poor response to proteasome inhibitor treatment in relapsed or refractory multiple myeloma patients [26][102]. In another approach, PA28α was reported as an accessible target for therapeutic antibodies against prostate cancer [116].
4. Proteasome and Immunoproteasome Inhibitors in Cancer Therapy
Given the importance of the proteasome in many aspects of carcinogenesis, targeting proteasomal subunits with small molecule inhibitors in tumor cells has emerged as an interesting avenue for cancer treatment [117]. Numerous proteasome inhibitors have been discovered in the last 30 years, which inhibit proteasomal activity through non-covalent or covalent bonding. These two groups further contain inhibitors belonging to different chemical classes such as aldehydes, boronates, epoxyketones, α-ketoaldehyde, β-lactones, vinyl-sulfones, syrbactins, and oxathiazolones [118][119][120][121].
The United States Food and Drug Administration (FDA) has approved three proteasome inhibitors, with the first being bortezomib for multiple myeloma [122]. Bortezomib is a reversible inhibitor that binds to both constitutive as well as the immunoproteasome [123][124]. Carfilzomib, a second-generation inhibitor approved in 2012, acts on both constitutive and inducible subunits with improved efficacy over bortezomib [125]. Ixazomib is an oral proteasome inhibitor that targets only constitutive proteasome subunits in a reversible manner [126]. Proteasome inhibitors have shown promising results in a clinical setting for treating hematological cancers such as multiple myeloma [127][128]. However, for solid tumors such as TNBC, prostate, and lung cancer, proteasomal inhibition has not demonstrated the same efficacy [129][130]. The lack of response in solid tumors might be due to insufficient potency or poor tumor penetration. In a previous study, although co-inhibition of β5i and β2i subunits can induce cell death in solid tumors, the required intratumoral concentration of proteasome inhibitors was not achieved [131]. Moreover, constitutive proteasome inhibition eventually results in the acquisition of chemoresistance by tumor cells. Though the mechanism of bortezomib resistance remains unclear, it had been demonstrated that immunoproteasomal inhibition in bortezomib-resistant cells can overcome tumor relapse [93]. Hence, studies which explore agents that can coordinately inhibit both the constitutive and immunoproteasome are required.
While bortezomib possesses β5i inhibitory activity, effective achievable intratumoral concentration, drug resistance, and off-target effects prevent immunoproteasomal inhibition [132]. Carfilzomib, with its potent chymotrypsin inhibitor activity, has a higher potential of achieving this co-inhibition [133]. In pre-clinical models, carfilzomib has shown broad anti-tumor activity against NSCLC and SCLC in a synergistic effect with cisplatin [134]. Specific immunoproteasome inhibitors (IPIs) are currently in development. PR-924 is a recently developed epoxyketone small molecule inhibitor that binds specifically to β5i [28]. PR-924 inhibited growth and proliferation of multiple myeloma cells in pre-clinical models and induced apoptosis in leukemia cell lines [28][135]. Another small molecule epoxyketone inhibitor, ONX-0914 (also known as PR-957) was found to be potent at targeting β5i and effective against bortezomib-resistant myeloma and colitis-induced colorectal cancer [136][137][138]. M3258 is a relatively new reversible inhibitor highly selective for β5i subunit. Orally bioavailable, this inhibitor has demonstrated significant efficacy in multiple myeloma xenograft models as well as higher anti-tumor activity compared to other non-selective IPIs like bortezomib in in-vivo settings. Thus, promising preclinical profile of M3258 has propelled its entry into phase I clinical trials [139][140].
Unlike the previous epoxyketone-derived IPIs, UK-101 was reported to selectively inhibit the catalytic β1i subunit and showed robust activity against prostate cancer in both in vitro and in vivo studies [141][142]. Another β1i inhibitor, IPSI-001 was found to be promising against myeloma [143]. Due to their high selectivity and lower toxicity, immunoproteasome-specific inhibitors have been touted as novel anti-cancer therapeutics. Interestingly, multiple myeloma-cells resistant to constitutive proteasome inhibitors have been shown to be better responders to IPI treatment when tumor cells were pre-exposed to them, which may indicate synergy of dual inhibition of constitutive and immunoproteasomes [144]. However, as IPIs show inhibition of both constitutive and immunoproteasome enzymatic activity, further study is required to evaluate the role of immunoproteasome inhibition alone [133][136][141]. Emergence of chemoresistance against IPIs also requires further investigation. These studies, while preliminary, highlight the potential of therapeutic targeting of immunoproteasome.
References
- Voges, D.; Zwickl, P.; Baumeister, W. The 26S Proteasome: A Molecular Machine Designed for Controlled Proteolysis. Annu. Rev. Biochem. 1999, 68, 1051–1068.
- Collins, G.A.; Goldberg, A.L. The Logic of the 26S Proteasome. Cell 2017, 169, 792–806.
- Unno, M.; Mizushima, T.; Morimoto, Y.; Tomisugi, Y.; Tanaka, K.; Yasuoka, N.; Tsukihara, T. The Structure of the Mammalian 20S Proteasome at 2.75 Å Resolution. Structure 2002, 10, 609–618.
- Rock, K.L.; Gramm, C.; Rothstein, L.; Clark, K.; Stein, R.; Dick, L.; Hwang, D.; Goldberg, A.L. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 1994, 78, 761–771.
- Aki, M.; Shimbara, N.; Takashina, M.; Akiyama, K.; Kagawa, S.; Tamura, T.; Tanahashi, N.; Yoshimura, T.; Tanaka, K.; Ichihara, A. Interferon-γ Induces Different Subunit Organizations and Functional Diversity of Proteasomes1. J. Biochem. 1994, 115, 257–269.
- Tanaka, K. The proteasome: Overview of structure and functions. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 12–36.
- Goldberg, A.L.; Cascio, P.; Saric, T.; Rock, K.L. The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol. Immunol. 2002, 39, 147–164.
- Rock, K.L.; Goldberg, A.L. Degradation of cell proteins and the generation of mhc class i-presented peptides. Annu. Rev. Immunol. 1999, 17, 739–779.
- Abele, R.; Tampé, R. The ABCs of Immunology: Structure and Function of TAP, the Transporter Associated with Antigen Processing. Physiology 2004, 19, 216–224.
- Pamer, E.; Cresswell, P. Mechanisms of mhc class i–restricted antigen processing. Annu. Rev. Immunol. 1998, 16, 323–358.
- Boes, B.; Hengel, H.; Ruppert, T.; Multhaup, G.; Koszinowski, U.H.; Kloetzel, P.M. Interferon gamma stimulation modulates the proteolytic activity and cleavage site preference of 20S mouse proteasomes. J. Exp. Med. 1994, 179, 901–909.
- Gaczynska, M.; Rock, K.L.; Goldberg, A.L. γ-Interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature 1993, 365, 264–267.
- Huber, E.M.; Basler, M.; Schwab, R.; Heinemeyer, W.; Kirk, C.J.; Groettrup, M.; Groll, M. Immuno- and Constitutive Proteasome Crystal Structures Reveal Differences in Substrate and Inhibitor Specificity. Cell 2012, 148, 727–738.
- Kincaid, E.Z.; Che, J.W.; York, I.; Escobar, H.; Reyes-Vargas, E.; Delgado, J.C.; Welsh, R.M.; Karow, M.L.; Murphy, A.J.; Valenzuela, D.M.; et al. Mice completely lacking immunoproteasomes show major changes in antigen presentation. Nat. Immunol. 2012, 13, 129–135.
- Winter, M.B.; La Greca, F.; Arastu-Kapur, S.; Caiazza, F.; Cimermancic, P.; Buchholz, T.J.; Anderl, J.L.; Ravalin, M.; Bohn, M.F.; Sali, A.; et al. Immunoproteasome functions explained by divergence in cleavage specificity and regulation. Elife 2017, 6, e27364.
- Mishto, M.; Liepe, J.; Textoris-Taube, K.; Keller, C.; Henklein, P.; Weberruß, M.; Dahlmann, B.; Enenkel, C.; Voigt, A.; Kuckelkorn, U.; et al. Proteasome isoforms exhibit only quantitative differences in cleavage and epitope generation. Eur. J. Immunol. 2014, 44, 3508–3521.
- Gutcher, I.; Becher, B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J. Clin. Investig. 2007, 117, 1119–1127.
- Kalim, K.W.; Basler, M.; Kirk, C.J.; Groettrup, M. Immunoproteasome Subunit LMP7 Deficiency and Inhibition Suppresses Th1 and Th17 but Enhances Regulatory T Cell Differentiation. J. Immunol. 2012, 189, 4182–4193.
- Cui, Z.; Hwang, S.M.; Gomes, A.V. Identification of the Immunoproteasome as a Novel Regulator of Skeletal Muscle Differentiation. Mol. Cell. Biol. 2014, 34, 96–109.
- Rouette, A.; Trofimov, A.; Haberl, D.; Boucher, G.; Lavallée, V.P.; D’Angelo, G.; Hébert, J.; Sauvageau, G.; Lemieux, S.; Perreault, C. Expression of immunoproteasome genes is regulated by cell-intrinsic and -extrinsic factors in human cancers. Sci. Rep. 2016, 6, 1–14.
- Lee, M.; Song, I.H.; Heo, S.H.; Kim, Y.A.; Park, I.A.; Bang, W.S.; Park, H.S.; Gong, G.; Lee, H.J. Expression of immunoproteasome subunit LMP7 in breast cancer and its association with immune-related markers. Cancer Res. Treat. 2019, 51, 80–89.
- Kiuchi, T.; Tomaru, U.; Ishizu, A.; Imagawa, M.; Iwasaki, S.; Suzuki, A.; Otsuka, N.; Ohhara, Y.; Kinoshita, I.; Matsuno, Y.; et al. Expression of the immunoproteasome subunit β5i in non-small cell lung carcinomas. J. Clin. Pathol. 2020, 74, 300–306.
- Kwon, C.H.; Park, H.J.; Choi, Y.R.; Kim, A.; Kim, H.W.; Choi, J.H.; Hwang, C.S.; Lee, S.J.; Choi, C.I.; Jeon, T.Y.; et al. PSMB8 and PBK as potential gastric cancer subtype-specific biomarkers associated with prognosis. Oncotarget 2016, 7, 21454.
- Liu, Y.; Yang, H.Z.; Jiang, Y.J.; Xu, L.Q. miR-451a is downregulated and targets PSMB8 in prostate cancer. Kaohsiung J. Med Sci. 2020, 36, 494–500.
- Wang, Q.; Pan, F.; Li, S.; Huang, R.; Wang, X.; Wang, S.; Liao, X.; Li, D.; Zhang, L. The prognostic value of the proteasome activator subunit gene family in skin cutaneous melanoma. J. Cancer 2019, 10, 2205–2219.
- Dytfeld, D.; Luczak, M.; Wrobel, T.; Usnarska-Zubkiewicz, L.; Brzezniakiewicz, K.; Jamroziak, K.; Giannopoulos, K.; Przybylowicz-Chalecka, A.; Ratajczak, B.; Czerwinska-Rybak, J.; et al. Comparative proteomic profiling of refractory/relapsed multiple myeloma reveals biomarkers involved in resistance to bortezomib-based therapy. Oncotarget 2016, 7, 56726–56736.
- Lou, S.; Cleven, A.H.G.; Balluff, B.; de Graaff, M.; Kostine, M.; Bruijn, I.B.; McDonnell, L.A.; Bovée, J.V.M.G. High nuclear expression of proteasome activator complex subunit 1 predicts poor survival in soft tissue leiomyosarcomas. Clin. Sarcoma Res. 2016, 6, 17.
- Singh, A.V.; Bandi, M.; Aujay, M.A.; Kirk, C.J.; Hark, D.E.; Raje, N.; Chauhan, D.; Anderson, K.C. PR-924, a selective inhibitor of the immunoproteasome subunit LMP-7, blocks multiple myeloma cell growth both in vitro and in vivo. Br. J. Haematol. 2011, 152, 155–163.
- Xu, H.; Ju, D.; Jarois, T.; Xie, Y. Diminished feedback regulation of proteasome expression and resistance to proteasome inhibitors in breast cancer cells. Breast Cancer Res. Treat. 2008, 107, 267–274.
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of Antigen Processing. Annu. Rev. Immunol. 2013, 31, 443–473.
- Eggensperger, S.; Tampé, R. The transporter associated with antigen processing: A key player in adaptive immunity. Biol. Chem. 2015, 396, 1059–1072.
- Martinez, C.K.; Monaco, J.J. Homology of proteasome subunits to a major histocompatibility complex-linked LMP gene. Nature 1991, 353, 664–667.
- Driscoll, J.; Brown, M.G.; Finley, D.; Monaco, J.J. MHC-linked LMP gene products specifically alter peptidase activities of the proteasome. Nature 1993, 365, 262–264.
- Akiyama, K.; Yokota, K.; Kagawa, S.; Shimbara, N.; Tamura, T.; Akioka, H.; Nothwang, H.; Noda, C.; Tanaka, K.; Ichihara, A. cDNA cloning and interferon gamma down-regulation of proteasomal subunits X and Y. Science 1994, 265, 1231–1234.
- Groll, M.; Ditzel, L.; Löwe, J.; Stock, D.; Bochtler, M.; Bartunik, H.D.; Huber, R. Structure of 20S proteasome from yeast at 2.4Å resolution. Nature 1997, 386, 463–471.
- DeMartino, G.N.; Slaughter, C.A. The Proteasome, a Novel Protease Regulated by Multiple Mechanisms. J. Biol. Chem. 1999, 274, 22123–22126.
- Ortiz-Navarrete, V.; Seelig, A.; Gernold, M.; Frentzel, S.; Kloetzel, P.M.; Hämmerling, G.J. Subunit of the “20S” proteasome (multicatalytic proteinase) encoded by the major histocompatibility complex. Nature 1991, 353, 662–664.
- Kelly, A.; Powis, S.H.; Glynne, R.; Radley, E.; Beck, S.; Trowsdale, J. Second proteasome-related gene in the human MHC class II region. Nature 1991, 353, 667–668.
- Rechsteiner, M.; Hill, C.P. Mobilizing the proteolytic machine: Cell biological roles of proteasome activators and inhibitors. Trends Cell Biol. 2005, 15, 27–33.
- Ahn, J.Y.; Tanahashi, N.; Akiyama, K.; Hisamatsu, H.; Noda, C.; Tanaka, K.; Chung, C.H.; Shibmara, N.; Willy, P.J.; Mott, J.D.; et al. Primary structures of two homologous subunits of PA28, a γ-interferon-inducible protein activator of the 20S proteasome. FEBS Lett. 1995, 366, 37–42.
- Tanahashi, N.; Yokota, K.; Ahn, J.Y.; Chung, C.H.; Fujiwara, T.; Takahashi, E.; DeMartino, G.N.; Slaughter, C.A.; Toyonaga, T.; Yamamura, K.; et al. Molecular properties of the proteasome activator PA28 family proteins and γ-interferon regulation. Genes Cells 1997, 2, 195–211.
- Marques, A.J.; Palanimurugan, R.; Matias, A.C.; Ramos, P.C.; Dohmen, R.J. Catalytic Mechanism and Assembly of the Proteasome. Chem. Rev. 2009, 109, 1509–1536.
- Hirano, Y.; Hendil, K.B.; Yashiroda, H.; Iemura, S.; Nagane, R.; Hioki, Y.; Natsume, T.; Tanaka, K.; Murata, S. A heterodimeric complex that promotes the assembly of mammalian 20S proteasomes. Nature 2005, 437, 1381–1385.
- Yashiroda, H.; Mizushima, T.; Okamoto, K.; Kameyama, T.; Hayashi, H.; Kishimoto, T.; Niwa, S.; Kasahara, M.; Kurimoto, E.; Sakata, E.; et al. Crystal structure of a chaperone complex that contributes to the assembly of yeast 20S proteasomes. Nat. Struct. Mol. Biol. 2008, 15, 228–236.
- Griffin, T.A.; Nandi, D.; Cruz, M.; Fehling, H.J.; Kaer, L.V.; Monaco, J.J.; Colbert, R.A. Immunoproteasome Assembly: Cooperative Incorporation of Interferon γ (IFN-γ)–inducible Subunits. J. Exp. Med. 1998, 187, 97–104.
- Groettrup, M.; Standera, S.; Stohwasser, R.; Kloetzel, P.M. The subunits MECL-1 and LMP2 are mutually required for incorporation into the 20S proteasome. Proc. Natl. Acad. Sci. USA 1997, 94, 8970–8975.
- Kingsbury, D.J.; Griffin, T.A.; Colbert, R.A. Novel Propeptide Function in 20 S Proteasome Assembly Influences β Subunit Composition. J. Biol. Chem. 2000, 275, 24156–24162.
- Heink, S.; Ludwig, D.; Kloetzel, P.-M.; Kruger, E. From The Cover: IFN- -induced immune adaptation of the proteasome system is an accelerated and transient response. Proc. Natl. Acad. Sci. USA 2005, 102, 9241–9246.
- Ramos, P.C.; Höckendorff, J.; Johnson, E.S.; Varshavsky, A.; Dohmen, R.J. Ump1p Is Required for Proper Maturation of the 20S Proteasome and Becomes Its Substrate upon Completion of the Assembly. Cell 1998, 92, 489–499.
- Früh, K.; Gossen, M.; Wang, K.; Bujard, H.; Peterson, P.A.; Yang, Y. Displacement of housekeeping proteasome subunits by MHC-encoded LMPs: A newly discovered mechanism for modulating the multicatalytic proteinase complex. EMBO J. 1994, 13, 3236–3244.
- Masters, E.I.; Pratt, G.; Förster, A.; Hill, C.P. Purification and analysis of recombinant 11S activators of the 20S proteasome: Trypanosoma brucei PA26 and human PA28 alpha, PA28 beta, and PA28 gamma. Methods Enzym. 2005, 398, 306–321.
- Cascio, P. Properties of the hybrid form of the 26S proteasome containing both 19S and PA28 complexes. EMBO J. 2002, 21, 2636–2645.
- Kopp, F.; Dahlmann, B.; Kuehn, L. Reconstitution of hybrid proteasomes from purified PA700–20 S complexes and PA28αβ activator: Ultrastructure and peptidase activities. J. Mol. Biol. 2001, 313, 465–471.
- Hendil, K.B.; Khan, S.; Tanaka, K. Simultaneous binding of PA28 and PA700 activators to 20 S proteasomes. Biochem. J. 1998, 332, 749–754.
- Guillaume, B.; Chapiro, J.; Stroobant, V.; Colau, D.; Holle, B.V.; Parvizi, G.; Bousquet-Dubouch, M.-P.; Theate, I.; Parmentier, N.; Van den Eynde, B.J. Two abundant proteasome subtypes that uniquely process some antigens presented by HLA class I molecules. Proc. Natl. Acad. Sci. USA 2010, 107, 18599–18604.
- Gaczynska, M.; Goldberg, A.L.; Tanaka, K.; Hendil, K.B.; Rock, K.L. Proteasome Subunits X and Y Alter Peptidase Activities in Opposite Ways to the Interferon-γ-induced Subunits LMP2 and LMP7. J. Biol. Chem. 1996, 271, 17275–17280.
- Orlowski, M.; Wilk, S. Catalytic Activities of the 20 S Proteasome, a Multicatalytic Proteinase Complex. Arch. Biochem. Biophys. 2000, 383, 1–16.
- Namiki, S.; Nakamura, T.; Oshima, S.; Yamazaki, M.; Sekine, Y.; Tsuchiya, K.; Okamoto, R.; Kanai, T.; Watanabe, M. IRF-1 mediates upregulation of LMP7 by IFN-γ and concerted expression of immunosubunits of the proteasome. FEBS Lett. 2005, 579, 2781–2787.
- Zhou, F. Molecular Mechanisms of IFN-γ to Up-Regulate MHC Class I Antigen Processing and Presentation. Int. Rev. Immunol. 2009, 28, 239–260.
- Chatterjee-Kishore, M.; Kishore, R.; Hicklin, D.J.; Marincola, F.M.; Ferrone, S. Different Requirements for Signal Transducer and Activator of Transcription 1α and Interferon Regulatory Factor 1 in the Regulation of Low Molecular Mass Polypeptide 2 and Transporter Associated with Antigen Processing 1 Gene Expression. J. Biol. Chem. 1998, 273, 16177–16183.
- Foss, G.S.; Larsen, F.; Solheim, J.; Prydz, H. Constitutive and interferon-γ-induced expression of the human proteasome subunit multicatalytic endopeptidase complex-like 11EMBL accession number for MECL1 cDNA: Y13640.1. Biochim. Biophys. Acta 1998, 1402, 17–28.
- Bose, S.; Brooks, P.; Mason, G.G.F.; Rivett, A.J. γ-Interferon decreases the level of 26 S proteasomes and changes the pattern of phosphorylation. Biochem. J. 2001, 353, 291–297.
- Jäkel, S.; Kuckelkorn, U.; Szalay, G.; Plötz, M.; Textoris-Taube, K.; Opitz, E.; Klingel, K.; Stevanovic, S.; Kandolf, R.; Kotsch, K.; et al. Differential Interferon Responses Enhance Viral Epitope Generation by Myocardial Immunoproteasomes in Murine Enterovirus Myocarditis. Am. J. Pathol. 2009, 175, 510–518.
- Shin, E.-C.; Seifert, U.; Kato, T.; Rice, C.M.; Feinstone, S.M.; Kloetzel, P.-M.; Rehermann, B. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J. Clin. Investig. 2006, 116, 3006–3014.
- Szalay, G.; Meiners, S.; Voigt, A.; Lauber, J.; Spieth, C.; Speer, N.; Sauter, M.; Kuckelkorn, U.; Zell, A.; Klingel, K.; et al. Ongoing Coxsackievirus Myocarditis Is Associated with Increased Formation and Activity of Myocardial Immunoproteasomes. Am. J. Pathol. 2006, 168, 1542–1552.
- Gavilán, M.P.; Castaño, A.; Torres, M.; Portavella, M.; Caballero, C.; Jiménez, S.; García-Martínez, A.; Parrado, J.; Vitorica, J.; Ruano, D. Age-related increase in the immunoproteasome content in rat hippocampus: Molecular and functional aspects. J. Neurochem. 2009, 108, 260–272.
- Kotamraju, S.; Matalon, S.; Matsunaga, T.; Shang, T.; Hickman-Davis, J.M.; Kalyanaraman, B. Upregulation of immunoproteasomes by nitric oxide: Potential antioxidative mechanism in endothelial cells. Free Radic. Biol. Med. 2006, 40, 1034–1044.
- Callahan, M.K.; Wohlfert, E.A.; Ménoret, A.; Srivastava, P.K. Heat Shock Up-Regulates lmp2 and lmp7 and Enhances Presentation of Immunoproteasome-Dependent Epitopes. J. Immunol. 2006, 177, 8393–8399.
- Ding, Q.; Martin, S.; Dimayuga, E.; Bruce-Keller, A.J.; Keller, J.N. LMP2 Knock-Out Mice Have Reduced Proteasome Activities and Increased Levels of Oxidatively Damaged Proteins. Antioxid. Redox Signal. 2006, 8, 130–135.
- Khan, M.A.S.; Oubrahim, H.; Stadtman, E.R. Inhibition of apoptosis in acute promyelocytic leukemia cells leads to increases in levels of oxidized protein and LMP2 immunoproteasome. Proc. Natl. Acad. Sci. USA 2004, 101, 11560–11565.
- Zhou, P.; Zanelli, E.; Smart, M.; David, C. Genomic Organization and Tissue Expression of Mouse Proteasome Gene Lmp-2. Genomics 1993, 16, 664–668.
- Wright, K.L.; White, L.C.; Kelly, A.; Beck, S.; Trowsdale, J.; Ting, J.P. Coordinate regulation of the human TAP1 and LMP2 genes from a shared bidirectional promoter. J. Exp. Med. 1995, 181, 1459–1471.
- James, A.B. Regulation of the Neuronal Proteasome by Zif268 (Egr1). J. Neurosci. 2006, 26, 1624–1634.
- Deshaies, R.J. Proteotoxic crisis, the ubiquitin-proteasome system, and cancer therapy. BMC Biol. 2014, 12, 94.
- Chen, L.; Brewer, M.D.; Guo, L.; Wang, R.; Jiang, P.; Yang, X. Enhanced Degradation of Misfolded Proteins Promotes Tumorigenesis. Cell Rep. 2017, 18, 3143–3154.
- Kumatori, A.; Tanaka, K.; Inamura, N.; Sone, S.; Ogura, T.; Matsumoto, T.; Tachikawa, T.; Shin, S.; Ichihara, A. Abnormally high expression of proteasomes in human leukemic cells. Proc. Natl. Acad. Sci. USA 1990, 87, 7071–7075.
- Petrocca, F.; Altschuler, G.; Tan, S.M.; Mendillo, M.L.; Yan, H.; Jerry, D.J.; Kung, A.L.; Hide, W.; Ince, T.A.; Lieberman, J. A Genome-wide siRNA Screen Identifies Proteasome Addiction as a Vulnerability of Basal-like Triple-Negative Breast Cancer Cells. Cancer Cell 2013, 24, 182–196.
- Morel, S.; Lévy, F.; Burlet-Schiltz, O.; Brasseur, F.; Probst-Kepper, M.; Peitrequin, A.-L.; Monsarrat, B.; Velthoven, R.V.; Cerottini, J.-C.; Boon, T.; et al. Processing of Some Antigens by the Standard Proteasome but Not by the Immunoproteasome Results in Poor Presentation by Dendritic Cells. Immunity 2000, 12, 107–117.
- Yang, B.-Y.; Song, J.-W.; Sun, H.; Xing, J.-C.; Yang, Z.-H.; Wei, C.-Y.; Xu, T.-Y.; Yu, Z.-N.; Zhang, Y.-N.; Wang, Y.-F.; et al. PSMB8 regulates glioma cell migration, proliferation, and apoptosis through modulating ERK1/2 and PI3K/AKT signaling pathways. Biomed. Pharmacother. 2018, 100, 205–212.
- Chen, N.X.; Liu, K.; Liu, X.; Zhang, X.X.; Han, D.Y. Induction and Regulation of the Immunoproteasome Subunit b5i (PSMB8) in Laryngeal and Hypopharyngeal Carcinoma Cells. Med. Sci. Monit. 2020, 26, e926110-1.
- Seliger, B.; Höhne, A.; Knuth, A.; Bernhard, H.; Ehring, B.; Tampé, R.; Huber, C. Reduced membrane major histocompatibility complex class I density and stability in a subset of human renal cell carcinomas with low TAP and LMP expression. Clin. Cancer Res. 1996, 2, 1427–1433.
- Vachharajani, N.; Joeris, T.; Luu, M.; Hartmann, S.; Pautz, S.; Jenike, E.; Pantazis, G.; Prinz, I.; Hofer, M.J.; Steinhoff, U.; et al. Prevention of colitis-associated cancer by selective targeting of immunoproteasome subunit LMP7. Oncotarget 2017, 8, 50447–50459.
- Piotrowska, Ż.; Niezgoda, M.; Młynarczyk, G.; Acewicz, M.; Kasacka, I. Comparative Assessment of the WNT/β-Catenin Pathway, CacyBP/SIP, and the Immunoproteasome Subunit LMP7 in Various Histological Types of Renal Cell Carcinoma. Front. Oncol. 2020, 10, 2530.
- Chang, H.H.; Cheng, Y.C.; Tsai, W.C.; Chen, Y. PSMB8 inhibition decreases tumor angiogenesis in glioblastoma through vascular endothelial growth factor A reduction. Cancer Sci. 2020, 111, 4142–4153.
- Hensley, S.E.; Zanker, D.; Dolan, B.P.; David, A.; Hickman, H.D.; Embry, A.C.; Skon, C.N.; Grebe, K.M.; Griffin, T.A.; Chen, W.; et al. Unexpected Role for the Immunoproteasome Subunit LMP2 in Antiviral Humoral and Innate Immune Responses. J. Immunol. 2010, 184, 4115–4122.
- Hayashi, T.; Faustman, D. Essential Role of Human Leukocyte Antigen-encoded Proteasome Subunits in NF-κB Activation and Prevention of Tumor Necrosis Factor-α-induced Apoptosis. J. Biol. Chem. 2000, 275, 5238–5247.
- Koerner, J.; Brunner, T.; Groettrup, M. Inhibition and deficiency of the immunoproteasome subunit LMP7 suppress the development and progression of colorectal carcinoma in mice. Oncotarget 2017, 8, 50873–50888.
- Leister, H.; Luu, M.; Staudenraus, D.; Krol, A.L.; Mollenkopf, H.-J.; Sharma, A.; Schmerer, N.; Schulte, L.N.; Bertrams, W.; Schmeck, B.; et al. Pro- and Antitumorigenic Capacity of Immunoproteasomes in Shaping the Tumor Microenvironment. Cancer Immunol. Res. 2021, 9, 682–692.
- Kalaora, S.; Lee, J.S.; Barnea, E.; Levy, R.; Greenberg, P.; Alon, M.; Yagel, G.; Eli, G.B.; Oren, R.; Peri, A.; et al. Immunoproteasome expression is associated with better prognosis and response to checkpoint therapies in melanoma. Nat. Commun. 2020, 11, 1–12.
- Tripathi, S.C.; Peters, H.L.; Taguchi, A.; Katayama, H.; Wang, H.; Momin, A.; Jolly, M.K.; Celiktas, M.; Rodriguez-Canales, J.; Liu, H.; et al. Immunoproteasome deficiency is a feature of non-small cell lung cancer with a mesenchymal phenotype and is associated with a poor outcome. Proc. Natl. Acad. Sci. USA 2016, 113, E1555–E1564.
- Shoji, T.; Kikuchi, E.; Kikuchi, J.; Takashima, Y.; Furuta, M.; Takahashi, H.; Tsuji, K.; Maeda, M.; Kinoshita, I.; Dosaka-Akita, H.; et al. Evaluating the immunoproteasome as a potential therapeutic target in cisplatin-resistant small cell and non-small cell lung cancer. Cancer Chemother. Pharmacol. 2020, 85, 843–853.
- Busse, A.; Kraus, M.; Na, I.K.; Rietz, A.; Scheibenbogen, C.; Driessen, C.; Blau, I.W.; Thiel, E.; Keilholz, U. Sensitivity of tumor cells to proteasome inhibitors is associated with expression levels and composition of proteasome subunits. Cancer 2008, 112, 659–670.
- Niewerth, D.; Kaspers, G.J.L.; Assaraf, Y.G.; van Meerloo, J.; Kirk, C.J.; Anderl, J.; Blank, J.L.; van de Ven, P.M.; Zweegman, S.; Jansen, G.; et al. Interferon-γ-induced upregulation of immunoproteasome subunit assembly overcomes bortezomib resistance in human hematological cell lines. J. Hematol. Oncol. 2014, 7, 7.
- Zhou, J.; Chng, W.-J. Novel mechanism of drug resistance to proteasome inhibitors in multiple myeloma. World J. Clin. Oncol. 2019, 10, 303–306.
- Adwal, A.; Croft, P.K.-D.; Shakya, R.; Lim, M.; Kalaw, E.; Taege, L.D.; Reed, A.E.M.; Lakhani, S.R.; Callen, D.F.; Saunus, J.M. Tradeoff between metabolic i-proteasome addiction and immune evasion in triple-negative breast cancer. Life Sci. Alliance 2020, 3, e201900562.
- Fan, X.; Zhao, Y. miR-451a inhibits cancer growth, epithelial-mesenchymal transition and induces apoptosis in papillary thyroid cancer by targeting PSMB8. J. Cell. Mol. Med. 2019, 23, 8067–8075.
- Yang, X.-W.; Wang, P.; Liu, J.-Q.; Zhang, H.; Xi, W.-D.; Jia, X.-H.; Wang, K.-K. Coordinated regulation of the immunoproteasome subunits by PML/RARα and PU.1 in acute promyelocytic leukemia. Oncogene 2014, 33, 2700–2708.
- Bruzzoni-Giovanelli, H.; González, J.R.; Sigaux, F.; Villoutreix, B.O.; Cayuela, J.M.; Guilhot, J.; Preudhomme, C.; Guilhot, F.; Poyet, J.-L.; Rousselot, P. Genetic polymorphisms associated with increased risk of developing chronic myelogenous leukemia. Oncotarget 2015, 6, 36269–36277.
- Mirandola, P.; Micheloni, C.; Solenghi, E.; Artico, M.; Soda, G.; Zanelli, G.; Pelusi, G.; Fiorini, T.; Cocco, L.; Vitale, M.; et al. Expression of HLA class I antigen and proteasome subunits LMP-2 and LMP-10 in primary vs. metastatic breast carcinoma lesions. Int. J. Oncol. 2004, 25, 1625–1629.
- Wang, C.; Cicek, M.S.; Charbonneau, B.; Kalli, K.R.; Armasu, S.M.; Larson, M.C.; Konecny, G.E.; Winterhoff, B.; Fan, J.B.; Bibikova, M.; et al. Tumor hypomethylation at 6p21.3 associates with longer time to recurrence of high-grade serous epithelial ovarian cancer. Cancer Res. 2014, 74, 3084–3091.
- Seliger, B.; Atkins, D.; Bock, M.; Ritz, U.; Ferrone, S.; Huber, C.; Störkel, S. Characterization of human lymphocyte antigen class I antigen-processing machinery defects in renal cell carcinoma lesions with special emphasis on transporter-associated with antigen-processing down-regulation. Clin. Cancer Res. 2003, 9, 1721–1727.
- Thompson, J.C.; Davis, C.; Deshpande, C.; Hwang, W.T.; Jeffries, S.; Huang, A.; Mitchell, T.C.; Langer, C.J.; Albelda, S.M. Gene signature of antigen processing and presentation machinery predicts response to checkpoint blockade in non-small cell lung cancer (NSCLC) and melanoma. J. ImmunoTherapy Cancer 2020, 8, e000974.
- Liew, P.L.; Huang, R.L.; Weng, Y.C.; Fang, C.L.; Huang, T.H.-M.; Lai, H.C. Distinct methylation profile of mucinous ovarian carcinoma reveals susceptibility to proteasome inhibitors. Int. J. Cancer 2018, 143, 355–367.
- Fellerhoff, B.; Gu, S.; Laumbacher, B.; Nerlich, A.G.; Weiss, E.H.; Glas, J.; Kopp, R.; Johnson, J.P.; Wank, R. The LMP7-K allele of the immunoproteasome exhibits reduced transcript stability and predicts high risk of colon cancer. Cancer Res. 2011, 71, 7145–7154.
- Li, C.; Dai, S.; Yan, Z.; Zhang, X.; Liu, S.; Wang, X.; Wang, J.; Shi, L.; Yao, Y. Genetic polymorphisms of proteasome subunit genes of the MHC-I antigen-presenting system are associated with cervical cancer in a Chinese Han population. Hum. Immunol. 2020, 81, 445–451.
- De Graaf, N.; van Helden, M.J.G.; Textoris-Taube, K.; Chiba, T.; Topham, D.J.; Kloetzel, P.M.; Zaiss, D.M.W.; Sijts, A.J.A.M. PA28 and the proteasome immunosubunits play a central and independent role in the production of MHC class I-binding peptides in vivo. Eur. J. Immunol. 2011, 41, 926–935.
- Sun, Y.; Sijts, A.J.A.M.; Song, M.; Janek, K.; Nussbaum, A.K.; Kral, S.; Schirle, M.; Stevanovic, S.; Paschen, A.; Schild, H.; et al. Expression of the proteasome activator PA28 rescues the presentation of a cytotoxic T lymphocyte epitope on melanoma cells. Cancer Res. 2002, 62, 2875–2882.
- Sijts, E.J.A.M.; Kloetzel, P.M. The role of the proteasome in the generation of MHC class I ligands and immune responses. Cell Mol. Life Sci. 2011, 68, 1491–1502.
- Cascio, P. PA28αβ: The enigmatic magic ring of the proteasome? Biomolecules 2014, 4, 566–584.
- Ossendorp, F.; Fu, N.; Camps, M.; Granucci, F.; Gobin, S.J.P.; van den Elsen, P.J.; Schuurhuis, D.; Adema, G.J.; Lipford, G.B.; Chiba, T.; et al. Differential Expression Regulation of the α and β Subunits of the PA28 Proteasome Activator in Mature Dendritic Cells. J. Immunol. 2005, 174, 7815–7822.
- Longuespée, R.; Boyon, C.; Castellier, C.; Jacquet, A.; Desmons, A.; Kerdraon, O.; Vinatier, D.; Fournier, I.; Day, R.; Salzet, M. The C-terminal fragment of the immunoproteasome PA28S (Reg alpha) as an early diagnosis and tumor-relapse biomarker: Evidence from mass spectrometry profiling. Histochem. Cell Biol. 2012, 138, 141–154.
- Feng, X.; Jiang, Y.; Xie, L.; Jiang, L.; Li, J.; Sun, C.; Xu, H.; Wang, R.; Zhou, M.; Zhou, Y.; et al. Overexpression of proteasomal activator PA28α serves as a prognostic factor in oral squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2016, 35, 35.
- Li, S.; Dai, X.; Gong, K.; Song, K.; Tai, F.; Shi, J. PA28α/β Promote Breast Cancer Cell Invasion and Metastasis via Down-Regulation of CDK15. Front. Oncol. 2019, 9, 1283.
- Chen, J.Y.; Xu, L.; Fang, W.M.; Han, J.Y.; Wang, K.; Zhu, K.S. Identification of PA28β as a potential novel biomarker in human esophageal squamous cell carcinoma. Tumor Biol. 2017, 39, 1010428317719780.
- Keller, M.; Ebstein, F.; Bürger, E.; Textoris-Taube, K.; Gorny, X.; Urban, S.; Zhao, F.; Dannenberg, T.; Sucker, A.; Keller, C.; et al. The proteasome immunosubunits, PA28 and ER-aminopeptidase 1 protect melanoma cells from efficient MART-126-35-specific T-cell recognition. Eur. J. Immunol. 2015, 45, 3257–3268.
- Sánchez-Martín, D.; Martínez-Torrecuadrada, J.; Teesalu, T.; Sugahara, K.N.; Alvarez-Cienfuegos, A.; Ximénez-Embún, P.; Fernández-Periáñez, R.; Martín, M.T.; Molina-Privado, I.; Ruppen-Cañás, I.; et al. Proteasome activator complex PA28 identified as an accessible target in prostate cancer by in vivo selection of human antibodies. Proc. Natl. Acad. Sci. USA 2013, 110, 13791–13796.
- Almond, J.B.; Cohen, G.M. The proteasome: A novel target for cancer chemotherapy. Leukemia 2002, 16, 433–443.
- Kisselev, A.F.; van der Linden, W.A.; Overkleeft, H.S. Proteasome Inhibitors: An Expanding Army Attacking a Unique Target. Chem. Biol. 2012, 19, 99–115.
- Kisselev, A.F.; Groettrup, M. Subunit specific inhibitors of proteasomes and their potential for immunomodulation. Curr. Opin. Chem. Biol. 2014, 23, 16–22.
- Ettari, R.; Zappalà, M.; Grasso, S.; Musolino, C.; Innao, V.; Allegra, A. Immunoproteasome-selective and non-selective inhibitors: A promising approach for the treatment of multiple myeloma. Pharmacol. Ther. 2018, 182, 176–192.
- Sherman, D.J.; Li, J. Proteasome Inhibitors: Harnessing Proteostasis to Combat Disease. Molecules 2020, 25, 671.
- Kane, R.C.; Bross, P.F.; Farrell, A.T.; Pazdur, R. Velcade ®: U.S. FDA Approval for the Treatment of Multiple Myeloma Progressing on Prior Therapy. Oncologist 2003, 8, 508–513.
- Adams, J.; Palombella, V.J.; Sausville, E.A.; Johnson, J.; Destree, A.; Lazarus, D.D.; Maas, J.; Pien, C.S.; Prakash, S.; Elliott, P.J. Proteasome Inhibitors: A Novel Class of Potent and Effective Antitumor Agents. Cancer Res. 1999, 59, 2615.
- Argyriou, A.A.; Bruna, J.; Marmiroli, P.; Cavaletti, G. Chemotherapy-induced peripheral neurotoxicity (CIPN): An update. Crit. Rev. Oncol. Hematol. 2012, 82, 51–77.
- Muchtar, E.; Gertz, M.A.; Magen, H. A practical review on carfilzomib in multiple myeloma. Eur. J. Haematol. 2016, 96, 564–577.
- Chauhan, D.; Tian, Z.; Zhou, B.; Kuhn, D.; Orlowski, R.; Raje, N.; Richardson, P.; Anderson, K.C. In Vitro and In Vivo Selective Antitumor Activity of a Novel Orally Bioavailable Proteasome Inhibitor MLN9708 against Multiple Myeloma Cells. Clin. Cancer Res. 2011, 17, 5311–5321.
- Kraus, M.; Bader, J.; Geurink, P.P.; Weyburne, E.S.; Mirabella, A.C.; Silzle, T.; Shabaneh, T.B.; van der Linden, W.A.; de Bruin, G.; Haile, S.R.; et al. The novel 2-selective proteasome inhibitor LU-102 synergizes with bortezomib and carfilzomib to overcome proteasome inhibitor resistance of myeloma cells. Haematologica 2015, 100, 1350–1360.
- Altun, M.; Galardy, P.J.; Shringarpure, R.; Hideshima, T.; LeBlanc, R.; Anderson, K.C.; Ploegh, H.L.; Kessler, B.M. Effects of PS-341 on the Activity and Composition of Proteasomes in Multiple Myeloma Cells. Cancer Res. 2005, 65, 7896–7901.
- Huang, Z.; Wu, Y.; Zhou, X.; Xu, J.; Zhu, W.; Shu, Y.; Liu, P. Efficacy of therapy with bortezomib in solid tumors: A review based on 32 clinical trials. Future Oncol. 2014, 10, 1795–1807.
- Dirix, L. A phase II study of the combination of endocrine treatment and bortezomib in patients with endocrine-resistant metastatic breast cancerA phase II study of the combination of endocrine treatment and bortezomib in patients with endocrine-resistant metastatic breast cancer. Oncol. Rep. 2011, 27, 657–663.
- Chen, D.; Frezza, M.; Schmitt, S.; Kanwar, J.; Dou, Q.P. Bortezomib as the First Proteasome Inhibitor Anticancer Drug: Current Status and Future Perspectives. Curr. Cancer Drug Targets 2011, 11, 239–253.
- Merin, N.; Kelly, K. Clinical Use of Proteasome Inhibitors in the Treatment of Multiple Myeloma. Pharmaceuticals 2014, 8, 1–20.
- Parlati, F.; Lee, S.J.; Aujay, M.; Suzuki, E.; Levitsky, K.; Lorens, J.B.; Micklem, D.R.; Ruurs, P.; Sylvain, C.; Lu, Y.; et al. Carfilzomib can induce tumor cell death through selective inhibition of the chymotrypsin-like activity of the proteasome. Blood 2009, 114, 3439–3447.
- Baker, A.F.; Hanke, N.T.; Sands, B.J.; Carbajal, L.; Anderl, J.L.; Garland, L.L. Carfilzomib demonstrates broad anti-tumor activity in pre-clinical non-small cell and small cell lung cancer models. J. Exp. Clin. Cancer Res. 2014, 33, 111.
- Niewerth, D.; van Meerloo, J.; Jansen, G.; Assaraf, Y.G.; Hendrickx, T.C.; Kirk, C.J.; Anderl, J.L.; Zweegman, S.; Kaspers, G.J.L.; Cloos, J. Anti-leukemic activity and mechanisms underlying resistance to the novel immunoproteasome inhibitor PR-924. Biochem. Pharmacol. 2014, 89, 43–51.
- Kim, K.B.; Myung, J.; Sin, N.; Crews, C.M. Proteasome inhibition by the natural products epoxomicin and dihydroeponemycin: Insights into specificity and potency. Bioorganic Med. Chem. Lett. 1999, 9, 3335–3340.
- Chauhan, D.; Singh, A.V.; Aujay, M.; Kirk, C.J.; Bandi, M.; Ciccarelli, B.; Raje, N.; Richardson, P.; Anderson, K.C. A novel orally active proteasome inhibitor ONX 0912 triggers in vitro and in vivo cytotoxicity in multiple myeloma. Blood 2010, 116, 4906–4915.
- Ruschak, A.M.; Slassi, M.; Kay, L.E.; Schimmer, A.D. Novel Proteasome Inhibitors to Overcome Bortezomib Resistance. JNCI J. Natl. Cancer Inst. 2011, 103, 1007–1017.
- Klein, M.; Busch, M.; Friese-Hamim, M.; Crosignani, S.; Fuchss, T.; Musil, D.; Rohdich, F.; Sanderson, M.P.; Seenisamy, J.; Walter-Bausch, G.; et al. Structure-Based Optimization and Discovery of M3258, a Specific Inhibitor of the Immunoproteasome Subunit LMP7 (β5i). J. Med. Chem. 2021, 64, 10230–10245.
- Sanderson, M.P.; Friese-Hamim, M.; Walter-Bausch, G.; Busch, M.; Gaus, S.; Musil, D.; Rohdich, F.; Zanelli, U.; Downey-Kopyscinski, S.L.; Mitsiades, C.S.; et al. M3258 Is a Selective Inhibitor of the Immunoproteasome Subunit LMP7 (β5i) Delivering Efficacy in Multiple Myeloma Models. Mol. Cancer 2021, 20, 1378–1387.
- Wehenkel, M.; Ban, J.-O.; Ho, Y.-K.; Carmony, K.C.; Hong, J.T.; Kim, K.B. A selective inhibitor of the immunoproteasome subunit LMP2 induces apoptosis in PC-3 cells and suppresses tumour growth in nude mice. Br. J. Cancer 2012, 107, 53–62.
- Ho, Y.K.; Bargagna-Mohan, P.; Wehenkel, M.; Mohan, R.; Kim, K.-B. LMP2-Specific Inhibitors: Chemical Genetic Tools for Proteasome Biology. Chem. Biol. 2007, 14, 419–430.
- Kuhn, D.J.; Hunsucker, S.A.; Chen, Q.; Voorhees, P.M.; Orlowski, M.; Orlowski, R.Z. Targeted inhibition of the immunoproteasome is a potent strategy against models of multiple myeloma that overcomes resistance to conventional drugs and nonspecific proteasome inhibitors. Blood 2009, 113, 4667–4676.
- Downey-Kopyscinski, S.; Daily, E.W.; Gautier, M.; Bhatt, A.; Florea, B.I.; Mitsiades, C.S.; Richardson, P.G.; Driessen, C.; Overkleeft, H.S.; Kisselev, A.F. An inhibitor of proteasome β2 sites sensitizes myeloma cells to immunoproteasome inhibitors. Blood Adv. 2018, 2, 2443–2451.
More
Information
Subjects:
Cell Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
29 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




