
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wenwen Xin | + 1339 word(s) | 1339 | 2021-12-29 09:23:55 | | | |
| 2 | Yvaine Wei | + 259 word(s) | 1598 | 2022-01-12 04:33:02 | | |
Video Upload Options
Human coronaviruses (HCoVs) are associated with a range of respiratory symptoms. The discovery of severe acute respiratory syndrome (SARS)-CoV, Middle East respiratory syndrome, and SARS-CoV-2 pose a significant threat to human health. The HCoV-MS method is a sensitive assay that combines multiplex PCR with matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS), to detect and differentiate seven HCoVs simultaneously.
1. Introduction
Coronaviruses (CoVs) are large, enveloped, positive-sense RNA viruses that cause respiratory diseases in a range of animals, including humans [1]. CoVs are divided into four genera, namely δ-CoVs, γ-CoVs, β-CoVs, and α-CoVs, among which β-CoVs and α-CoVs can infect mammals [2]. Seven human CoVs (HCoVs) have been identified, including HCoV-NL63, HCoV-229E, HCoV-OC43, HCoV-HKU1, SARS-CoV, MERS-CoV, and SARS-CoV-2 [1][3]. Respiratory diseases caused by HCoV infection range from mild to severe. Approximately 15–30% of respiratory tract infections worldwide each year are caused by HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1. They are mild and self-healing diseases that do not pose a major threat to public health [4].
2. Performance of the Hcov-MS Method
3. Specificity of the HCoV-MS Method
4. Sensitivity of the HCoV-MS Method
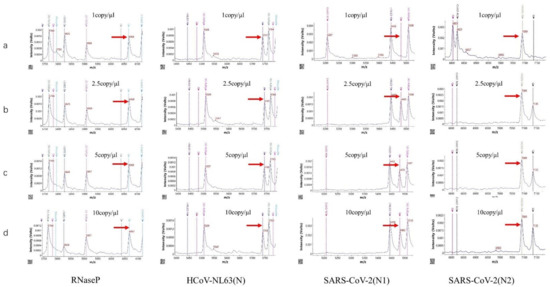
| Assays | Target | Detection Limit (Copies/Reaction) |
|---|---|---|
| Human RNase P | Human RNase P | 1 |
| HCoV-NL63 | N | 1 |
| RdRp | 1 | |
| HCoV-229E | N | 2.5 |
| RdRp | 2.5 | |
| HCoV-OC43 | N | 2.5 |
| RdRp | 2.5 | |
| HCoV-HKU1 | N | 1 |
| RdRp | 2.5 | |
| MERS-CoV | N | 1 |
| RdRp | 2.5 | |
| E | 2.5 | |
| ORF1b | 2.5 | |
| SARS-CoV | E | 5 |
| N | 5 | |
| ORF1b | 5 | |
| SARS-CoV-2 | N1 | 2.5 |
| N2 | 2.5 | |
| S | 2.5 | |
| ORF1b | 2.5 |
5. Sensitivity Comparisons of HCoV-MS and RT-PCR
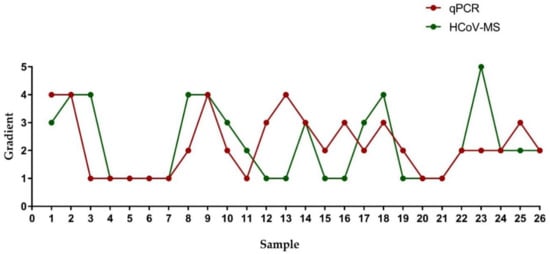
| Sample | Gradient | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||||
| RT-PCR * | HCoV-MS * | RT-PCR | HCoV-MS | RT-PCR | HCoV-MS | RT-PCR | HCoV-MS | RT-PCR | HCoV-MS | |
| 1 | No Ct/No Ct | − 1 | No Ct/No Ct | − | 40.74/No Ct | + 2 | 36.87/No Ct | + | 34.17/No Ct | + |
| 2 | No Ct/No Ct | − | No Ct/No Ct | − | 40.65/No Ct | − | 36.30/No Ct | + | 34.36/No Ct | + |
| 3 | 35.94/No Ct | − | 32.84/No Ct | − | 33.35/No Ct | − | 32.56/No Ct | + | 30.16/No Ct | + |
| 4 | 36.88/34.01 | + | 35.96/32.03 | + | 32.92/29.77 | + | 31.24/27.63 | + | 28.52/25.92 | + |
| 5 | 39.64/33.68 | + | 37.01/32.01 | + | 34.79/30.05 | + | 33.03/27.92 | + | 30.6/25.83 | + |
| 6 | 37.33/35.04 | + | 36.67/32.81 | + | 35.45/31.17 | + | 34.28/29.21 | + | 32.9/27.14 | + |
| 7 | 40.63/34.79 | + | 40.11/32.60 | + | 38.66/30.97 | + | 35.83/28.46 | + | 33.83/26.86 | + |
| 8 | No Ct/No Ct | − | No Ct/39.02 | − | 39.35/36.45 | − | 36.3/34.71 | + | 35.15/32.30 | + |
| 9 | No Ct/No Ct | − | No Ct/No Ct | − | No Ct/No Ct | − | 39.33/36.50 | + | 36.32/34.60 | + |
| 10 | No Ct/No Ct | − | 37.23/37.43 | − | 37.06/34.76 | + | 35.16/32.90 | + | 33.24/30.99 | + |
| 11 | 39.02/36.78 | − | 36.35/33.84 | + | 34.35/31.44 | + | 32.36/30.16 | + | 30.66/28.16 | + |
| 12 | No Ct/No Ct | + | No Ct/No Ct | + | No Ct/38.39 | + | 35.95/35.15 | + | 34.61/32.06 | + |
| 13 | No Ct/No Ct | + | No Ct/No Ct | + | No Ct/No Ct | + | 34.99/No Ct | + | 33.94/38.01 | + |
| 14 | No Ct/No Ct | − | No Ct/No Ct | − | No Ct/38.16 | + | 37.12/35.66 | + | 34.52/33.22 | + |
| 15 | No Ct/No Ct | + | 37.15/36.10 | + | 37.04/33.67 | + | 35.26/31.76 | + | 33.01/30.57 | + |
| 16 | No Ct/No Ct | + | 40.81/No Ct | + | No Ct/38.45 | + | 39.89/36.62 | + | 37.84/35.04 | + |
| 17 | No Ct/No Ct | − | No Ct/39.73 | − | 39.31/37.82 | + | 37.87/36.36 | + | 36.43/34.64 | + |
| 18 | No Ct/No Ct | − | No Ct/40.08 | − | No Ct/39.48 | − | 42.18/38.98 | + | 40.41/36.18 | + |
| 19 | No Ct/No Ct | + | 37.22/36.00 | + | 36.90/34.46 | + | 34.81/33.69 | + | 32.92/31.26 | + |
| 20 | 37.80/32.63 | + | 36.22/30.56 | + | 33.91/28.28 | + | 32.24/26.46 | + | 29.53/23.98 | + |
| 21 | 39.93/34.69 | + | 36.10/32.38 | + | 32.28/30.01 | + | 30.51/27.60 | + | 27.69/25.37 | + |
| 22 | No Ct/No Ct | − | No Ct/38.56 | + | 38.56/39.32 | + | 37.10/34.75 | + | 36.13/33.54 | + |
| 23 | No Ct/No Ct | − | 35.15/No Ct | − | 34.93/No Ct | − | 34.15/38.72 | − | 30.79/35.09 | + |
| 24 | No Ct/No Ct | − | 39.17/36.95 | + | 36.61/34.35 | + | 35.95/31.70 | + | 33.32/29.73 | + |
| 25 | No Ct/No Ct | − | No Ct/No Ct | + | No Ct/38.88 | + | 36.66/37.20 | + | 33.94/35.70 | + |
| 26 | No Ct/No Ct | − | 36.46/No Ct | + | 34.97/38.94 | + | 35.39/35.67 | + | 33.65/34.16 | + |
6. Research Findings of the HCoV-MS Method
A HCoV-MS method was established to simultaneously detect seven HcoVs, which can be used as a detection system when new HCoVs appear. Notably, the detection sensitivity of HCoV-MS is 1–5 copies/reaction. Except for SARS-CoV, other HCoVs could be detected with 1–2.5 copies/reaction. HCoV-NL63 was even detected with 1 copy/reaction. This sensitivity of this method outperforms that of other detection methods [20][21][22][23][24].
When testing 151 unknown clinical samples, the specificity and sensitivity of the HCoV-MS method reached 100%, surpassing other methods [25][26][27]. This could, however, be due to insufficient clinical samples. Samples of human/animal throat swabs or cell cultures were obtained in cooperation with UN-CoV-2020. Seven samples were positive, including four SARS-CoV-2 samples, two SARS-CoV samples, and one HCoV-NL63 sample. Moreover, the concentration of individual positive samples was low, but could still be accurately identified, which further highlights the detection ability of the HCoV-MS method.
The HCoV-MS method is high throughput, as reflected in the detection of multiple target genes and the requirement of small sample sizes. The HCoV-MS method could simultaneously detect 384 targets in one run spanning 30 min, with results automatically determined by the relevant software. Moreover, the reagent cost of the HCoV-MS method is relatively low, making this method ideal for large-scale population screening.
The HCoV-MS also has some limitations. This method is difficult to identify new HCoV because it is based on comparing and analyzing known HCoV sequences, selecting gene fragments with conserved intraspecies specificity. In conclusion, the HCoV-MS method has the characteristics of high throughput, speed, and sensitivity, only requiring a small number of samples. Therefore, it is expected to be a supplement to real-time PCR technology.
References
- Xiu, L.; Zhang, C.; Wu, Z.; Peng, J. Establishment and Application of a Universal Coronavirus Screening Method Using MALDI-TOF Mass Spectrometry. Front. Microbiol. 2017, 8, 1510.
- Ezhilan, M.; Suresh, I.; Nesakumar, N. SARS-CoV, MERS-CoV and SARS-CoV-2: A Diagnostic Challenge. Measurement 2021, 168, 108335.
- Fung, T.S.; Liu, D.X. Human Coronavirus: Host-Pathogen Interaction. Annu. Rev. Microbiol. 2019, 73, 529–557.
- Miller, K.; McGrath, M.E.; Hu, Z.; Ariannejad, S.; Weston, S.; Frieman, M.; Jackson, W.T. Coronavirus interactions with the cellular autophagy machinery. Autophagy 2020, 16, 2131–2139.
- Xiao-Shuang, Z.; Zeng, L.-P.; Yang, X.-L.; Ge, X.-Y.; Zhang, W.; Lin-Fa, W.; Xie, J.-Z.; Dong-Sheng, L.; Zhang, Y.-Z.; Wang, N.; et al. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017, 13, e1006698.
- Loeffelholz, M.J.; Tang, Y.-W. Laboratory diagnosis of emerging human coronavirus infections–the state of the art. Emerg. Microbes Infect. 2020, 9, 747–756.
- Gu, W.; Miller, S.; Chiu, C.Y. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 319–338.
- Gao, J.; Quan, L. Current Status of Diagnostic Testing for SARS-CoV-2 Infection and Future Developments: A Review. Med. Sci. Monit. 2020, 26.
- Appak, Ö.; Duman, M.; Belet, N.; Sayiner, A.A. Viral respiratory infections diagnosed by multiplex polymerase chain reaction in pediatric patients. J. Med. Virol. 2019, 91, 731–737.
- Gilsenan-Reed, C.; Higgins, G.; Langlois, N. Determining a sampling regime for PCR detection of respiratory tract viral infection at coronial post-mortem examinations. Forensic Sci. Med. Pathol. 2020, 16, 457–462.
- Pabbaraju, K.; Wong, A.A.; Ma, R.; Zelyas, N.; Tipples, G.A. Development and validation of a multiplex reverse transcriptase-PCR assay for simultaneous testing of influenza A, influenza B and SARS-CoV-2. J. Virol. Methods 2021, 293, 114151.
- Li, J.; Mao, N.-Y.; Zhang, C.; Yang, M.-J.; Wang, M.; Xu, W.-B.; Ma, X.-J. The development of a GeXP-based multiplex reverse transcription-PCR assay for simultaneous detection of sixteen human respiratory virus types/subtypes. BMC Infect. Dis. 2012, 12, 189.
- Vogels, C.B.F.; Brito, A.F.; Wyllie, A.L.; Fauver, J.R.; Ott, I.M.; Kalinich, C.C.; Petrone, M.E.; Casanovas-Massana, A.; Muenker, M.C.; Moore, A.J.; et al. Analytical sensitivity and efficiency comparisons of SARS-CoV-2 RT–qPCR primer–probe sets. Nat. Microbiol. 2020, 5, 1299–1305.
- Nörz, D.; Hoffmann, A.; Aepfelbacher, M.; Pfefferle, S.; Lütgehetmann, M. Clinical evaluation of a fully automated, laboratory-developed multiplex RT-PCR assay integrating dual-target SARS-CoV-2 and influenza A/B detection on a high-throughput platform. J. Med. Microbiol. 2021, 70, 001295.
- Settanni, L.; Corsetti, A. The use of multiplex PCR to detect and differentiate food- and beverage-associated microorganisms: A review. J. Microbiol. Methods 2007, 69, 1–22.
- Tsuchida, S.; Umemura, H.; Nakayama, T. Current Status of Matrix-Assisted Laser Desorption/Ionization–Time-of-Flight Mass Spectrometry (MALDI-TOF MS) in Clinical Diagnostic Microbiology. Molecules 2020, 25, 4775.
- Liu, N.; Wang, L.; Cai, G.; Zhang, D.; Lin, J. Establishment of a simultaneous detection method for ten duck viruses using MALDI-TOF mass spectrometry. J. Virol. Methods 2019, 273, 113723.
- Zhao, F.; Zhang, J.; Wang, X.; Liu, L.; Gong, J.; Zhai, Z.; He, L.; Meng, F.; Xiao, D. A multisite SNP genotyping and macrolide susceptibility gene method for Mycoplasma pneumoniae based on MALDI-TOF MS. iScience 2021, 24, 102447.
- Zhang, C.; Xiao, Y.; Du, J.; Ren, L.; Wang, J.; Peng, J.; Jin, Q. Application of Multiplex PCR Coupled with Matrix-Assisted Laser Desorption Ionization–Time of Flight Analysis for Simultaneous Detection of 21 Common Respiratory Viruses. J. Clin. Microbiol. 2015, 53, 2549–2554.
- Leung, E.C.-M.; Chow, V.C.-Y.; Lee, M.K.-P.; Tang, K.P.-S.; Li, D.K.-C.; Lai, R.W.-M. Evaluation of the Xpert Xpress SARS-CoV-2/Flu/RSV Assay for Simultaneous Detection of SARS-CoV-2, Influenza A and B Viruses, and Respiratory Syncytial Virus in Nasopharyngeal Specimens. J. Clin. Microbiol. 2021, 59, e02965-20.
- Paradis, S.; Lockamy, E.; Cooper, C.K.; Young, S. Clinical evaluation of the molecular-based BD SARS-CoV-2/flu for the BD MAX™ System. J. Clin. Virol. 2021, 143, 104946.
- Xi, Y.; Xu, C.-Z.; Xie, Z.-Z.; Zhu, D.-L.; Dong, J.-M.; Xiao, G. Development of a reverse transcription recombinase polymerase amplification assay for rapid detection of human respiratory syncytial virus. Mol. Cell. Probes 2019, 45, 8–13.
- Zhang, W.S.; Pan, J.; Li, F.; Zhu, M.; Xu, M.; Zhu, H.; Yu, Y.; Su, G. Reverse Transcription Recombinase Polymerase Amplification Coupled with CRISPR-Cas12a for Facile and Highly Sensitive Colorimetric SARS-CoV-2 Detection. Anal. Chem. 2021, 93, 4126–4133.
- Lin, Y.; Fu, Y.; Xu, M.; Su, L.; Cao, L.; Xu, J.; Cheng, X. Evaluation of a PCR/ESI-MS platform to identify respiratory viruses from nasopharyngeal aspirates. J. Med Virol. 2015, 87, 1867–1871.
- Aoki, A.; Mori, Y.; Okamoto, Y.; Jinno, H. Development of a genotyping platform for SARS-CoV-2 variants using high-resolution melting analysis. J. Infect. Chemother. 2021, 27, 1336–1341.
- Lope, P.; Maribel, H.; Egma, M.; Henri, B.; Carlos, P. Characterization of influenza A(H1N1)pdm09 isolates of Peru using HRM, a post PCR molecular biology method. Bioinformation 2019, 15, 640–645.
- Zhou, H.; Zhao, M.; Li, X.; Zhang, D.; Chen, C.; Feng, Z.; Ma, X. Clinical evaluation of the isothermal amplification assays for the detection of four common respiratory viruses in children with pneumonia. Arch. Virol. 2017, 162, 1311–1318.




