
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sebastiano A.G. Lava | + 1075 word(s) | 1075 | 2021-12-03 06:09:26 | | | |
| 2 | Lindsay Dong | Meta information modification | 1075 | 2022-01-12 04:42:09 | | |
Video Upload Options
Deferasirox is a first-line therapy for iron overload that can sometimes cause kidney damage. A proximal tubulopathy pattern may be observed on treatment with deferasirox. Since deferasirox-associated kidney damage is dose-dependent, physicians should prescribe the lowest efficacious dose.
1. Introduction
2. Kidney Tubular Damage Secondary to Deferasirox
The majority (61%) of the 57 patients were ≤18 years of age (Table 1). Three-quarters of the cases were affected by a thalassemia syndrome. Laboratory features consistent with kidney damage were mostly observed >6 months after starting a standard dose deferasirox therapy, although this information was not available in more than half of the cases. A recurrence of the kidney damage was noted in nine of the 18 patients, who were again exposed to deferasirox (usually in a reduced dose).
| Gender | ||
|---|---|---|
| Female, N (%) | 27 | 47 |
| Male, N (%) | 30 | 53 |
| Age | ||
| Years, median [interquartile range] | 15 [6.7–21] | |
| ≤18 years, N (%) | 35 | 61 |
| Underlying transfusion-dependent disease | ||
| Thalassemia syndrome, N (%) | 46 | 81 |
| Diamond Blackfan anemia, N (%) | 5 | 8.8 |
| Allogenic stem cell transplantation, N (%) | 3 | 5.3 |
| Other conditions ◆, N (%) | 3 | 5.3 |
| Deferasirox dose☩ | ||
| 20–30 mg/kg/day, N (%) | 46 | 87 |
| 31–42 mg/kg/day, N (%) | 7 | 13 |
| Duration of deferasirox therapy ✙ | ||
| ≤1 month, N (%) | 5 | 20 |
| 2–6 months, N (%) | 4 | 16 |
| >6 months, N (%) | 16 | 64 |
| Time to recovery after therapy withdrawal * | ||
| Information not given, N (%) | 37 | 65 |
| ≤1 week, N (%) | 2 | 3.5 |
| 2–4 weeks, N (%) | 3 | 5.3 |
| >6 months, N (%) | 5 | 8.8 |
| Persistent abnormalities reported | 2 | 3.5 |
| Deferasirox therapy rechallenge, N (%) | 18 | 32 |
| Relapse of kidney damage, N (%) | 9 | 16 |
| All | Tubulopathy without Kidney Injury |
Tubulopathy with Kidney Injury * |
||
|---|---|---|---|---|
| Latent | Overt | |||
| N | 57 | 11 | 37 | 9 |
| Age, years (median and IQR) | 15 [6.7–21] | 14 [11–19] | 11 [5.6–20] | 20 [18–33] |
| Females/males, N | 27/30 | 6/5 | 21/16 | 0/9 ✙ |
| Abnormal urinary findings, N | 54 | 11 | 34 | 9 |
| Renal glucosuria, N | 34 | 2 | 23 | 9 |
| Tubular proteinuria ☩, N | 21 | 8 | 16 | 2 |
| Excessive total proteinuria, N | 17 | 1 | 11 | 5 |
| Generalized aminoaciduria, N | 9 | 1 | 4 | 4 |
| Electrolyte-acid–base disorders, N | 46 | - | 37 | 9 |
| Metabolic acidosis, N | 38 ✿ | - | 31 | 7 |
| Hypophosphatemia, N | 35 | - | 27 | 8 |
| Hypokalemia, N | 24 | - | 18 | 6 |
| Hypouricemia, N | 11 | - | 7 | 4 |
| Hypocalcemia, N | 6 | - | 6 | 0 |
| Hyponatremia, N | 3 | - | 1 | 2 |
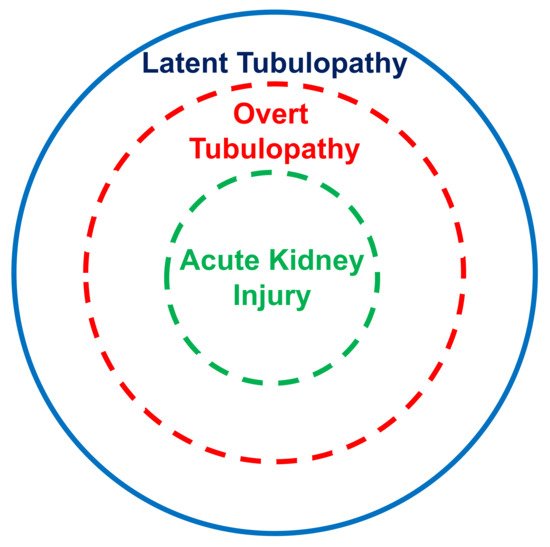
The kidney tubular damage associated with oral deferasirox therapy may present in three ways: (a) abnormal urinary findings consistent with latent tubular damage; (b) overt acid–base or electrolyte abnormalities; and (c) acute kidney injury (always associated with abnormal urinary findings and with an electrolyte or acid–base imbalance).
3. Conclusions
References
- Cappellini, M.D. Long-term efficacy and safety of deferasirox. Blood Rev. 2008, 22, S35–S41.
- Cappellini, M.D.; Pattoneri, P. Oral Iron Chelators. Annu. Rev. Med. 2009, 60, 25–38.
- Díaz-García, J.D.; Gallegos-Villalobos, A.; González-Espinoza, L.; Sanchez-Niño, M.D.; Villarrubia, J.; Ortiz, A.; Arduan, A.O. Deferasirox nephrotoxicity—The knowns and unknowns. Nat. Rev. Nephrol. 2014, 10, 574–586.
- Yui, J.C.; Geara, A.; Sayani, F. Deferasirox-associated Fanconi syndrome in adult patients with transfusional iron overload. Vox Sang. 2021, 116, 793–797.
- Hall, A.M.; Bass, P.; Unwin, R.J. Drug-induced renal Fanconi syndrome. QJM Int. J. Med. 2014, 107, 261–269.
- Scoglio, M.; Bronz, G.; Rinoldi, P.; Faré, P.; Betti, C.; Bianchetti, M.; Simonetti, G.; Gennaro, V.; Renzi, S.; Lava, S.; et al. Electrolyte and Acid-Base Disorders Triggered by Aminoglycoside or Colistin Therapy: A Systematic Review. Antibiotics 2021, 10, 140.
- Maggio, A.; Kattamis, A.; Felisi, M.; Reggiardo, G.; El-Beshlawy, A.; Bejaoui, M.; Sherief, L.; Christou, S.; Cosmi, C.; Della Pasqua, O.; et al. Evaluation of the efficacy and safety of deferiprone compared with deferasirox in paediatric patients with transfusion-dependent haemoglobinopathies (DEEP-2): A multicentre, randomised, open-label, non-inferiority, phase 3 trial. Lancet Haematol. 2020, 7, e469–e478.
- Gottwald, E.M.; Schuh, C.D.; Drücker, P.; Haenni, D.; Pearson, A.; Ghazi, S.; Bugarski, M.; Polesel, M.; Duss, M.; Landau, E.M.; et al. The iron chelator Deferasirox causes severe mitochondrial swelling without depolarization due to a specific effect on inner membrane permeability. Sci. Rep. 2020, 10, 1–15.
- Kattamis, A. Renal function abnormalities and deferasirox. Lancet Child Adolesc. Health 2018, 3, 2–3.
- Allegra, S.; De Francia, S.; Cusato, J.; Arduino, A.; Massano, D.; Longo, F.; Piga, A.; D’Avolio, A. Deferasirox pharmacogenetic influence on pharmacokinetic, efficacy and toxicity in a cohort of pediatric patients. Pharmacogenomics 2017, 18, 539–554.
- Liem, R.I.; Lanzkron, S.; Coates, T.D.; DeCastro, L.; Desai, A.; Ataga, K.I.; Cohen, R.T.; Haynes, J.J.; Osunkwo, I.; Lebensburger, J.D.; et al. American Society of Hematology 2019 guidelines for sickle cell disease: Cardiopulmonary and kidney disease. Blood Adv. 2019, 3, 3867–3897.




