
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Timothy Tse | + 2991 word(s) | 2991 | 2021-11-23 09:57:01 | | | |
| 2 | Lindsay Dong | + 223 word(s) | 3214 | 2022-01-12 07:08:59 | | |
Video Upload Options
Most ethanol is produced by Saccharomyces cerevisiae (yeast) fermentation of either crops rich in sucrose (e.g., sugar cane and sugar beet) or starch-rich crops (e.g., corn and starchy grains). Ethanol produced from these sources is termed a first-generation biofuel. Yeast fermentation can yield a range of additional valuable co-products that accumulate during primary fermentation (e.g., protein concentrates, water soluble metabolites, fusel alcohols, and industrial enzymes). Distillers’ solubles is a liquid co-product that can be used in animal feed or as a resource for recovery of valuable materials. In some processes it is preferred that this fraction is modified by a second fermentation with another fermentation organism (e.g., lactic acid bacteria). Such two stage fermentations can produce valuable compounds, such as 1,3-propanediol, organic acids, and bacteriocins. The use of lactic acid bacteria can also lead to the aggregation of stillage proteins and enable protein aggregation into concentrates. Once concentrated, the protein has utility as a high-protein feed ingredient. After separation of protein concentrates the remaining solution is a potential source of several known small molecules.
1. Introduction
| Co-Products | Market Size (US Dollars) |
Project Compound Annual Growth Rate (CAGR) |
|---|---|---|
| Ethanol | 89.1 billion (2019) | 4.8% by 2027 |
| Acetic acid | 8.92 billion (2019) | 5.2% by 2027 |
| Succinic acid | 181.6 million (2019) | 9.2% by 2022 |
| Lactic acid | 2.7 billion (2020) | 8.0% by 2028 |
| Glycerol | 2.6 billion (2019) | 4.0% by 2027 |
| Nootropics | 2.42 billion (2020) | 12.7% by 2028 |
| Dried distillers’ grains with solubles | 112.5 million (2020) | 5.2% by 2026 |
2. Thin Stillage and Distillers’ Grains
Following ethanolic fermentation and distillation processes, the by-product stillage contains much of the protein, oil, fiber, and non-starch carbohydrate that were not available to the yeast during fermentation. A common process for using these components starts with the separation of whole stillage into a liquid portion with suspended solids (thin stillage) and wet solids (distillers’ wet grains), using centrifugation, vibratory separation, or a press [22][23]. These by-products can then be further processed (i.e., drying and fractionation) or extracted into specific components.
Without any further processing, all or a portion of thin stillage can be returned or backset to the next fermentation. This practice replaces some of the water required to soak incoming feedstocks intended for fermentation [24]. Protein is a major nutrient remaining in the stillage, and many methods have been developed to recover stillage proteins. Grain thin stillage contains approximately 37% protein (w/w dry basis) [25], and research has been conducted to develop stillage protein concentrates. Physical clarification techniques using additives [26][27], gas flotation [28][29], centrifugation [30], and filtration [31][32] have been tested as approaches to produce protein-enriched solids from thin stillage. Another approach for improving stillage protein quality and concentration is through a two-stage fermentation strategy [14][33], where it is also possible to upgrade glycerol to higher value compounds. Where thin stillage is not suited for use as a feed, it may be used as a nitrogen-rich fertilizer for crops [34]. Another strategy is to pair thin stillage valorization with a protein extraction process. Protein extraction from oilseed meal requires the use of large volumes of solution to dissolve proteins before precipitation. Thin stillage has some dissolved protein, but it can be used as a solution for protein extraction from oilseed meal [35]. An economical use of thin stillage that avoids the need for evaporation while providing the benefit of the stillage as a nutrient solution involves simply providing stillage in the water for cattle. In this way, the stillage becomes a nutrient-rich water source [36].
Once thin stillage is separated by dewatering distillers’ wet grains [37][38], the thin stillage can then be dried to a concentrated syrup called distillers’ solubles (DS), which is useful as an animal feed component. Remaining solids or distillers’ wet grains (DWG) can be dried to produce distillers’ dried grains (DDG) for storage and shipping. An alternative practice is to add DS to the grains as they dry to produce dried distillers’ grains with solubles (DDGS), a product that is commonly used with cattle feed [39]. Compared to wet feed products, DDG and DDGS have extended shelf-life and are more easily shipped. The sale of fermentation by-products for use as cattle feed generally provides 10–20% of the total revenue of ethanol production facilities [40] while avoiding revenue losses that would be incurred if co-product disposal was necessary. Fractionation of the DDGS can concentrate protein and generate fractions with high fiber contents to produce additional protein and fiber products [41]. In some rations, the higher fiber content of DDGS is undesirable. Producing a higher protein- and fat-content feed ingredient can improve the value of this product stream. In addition to the use of stillage products in animal feed, a portion DDGS proteins can be more readily solubilized and extracted for a wide variety of industrial uses (e.g., biopolymer production) [42].
DDG, produced during first-generation biofuels processes, can be used as a substrate for a second fermentation after pre-treatment that converts unhydrolyzed and unprocessed cellulose into fermentable sugars [43].
Finally, oil is another product of bioethanol production that is often poorly utilized. For corn, methods of oil extraction from thin stillage have been patented [44], and other oil extraction methods from corn DDGS have been developed [45].
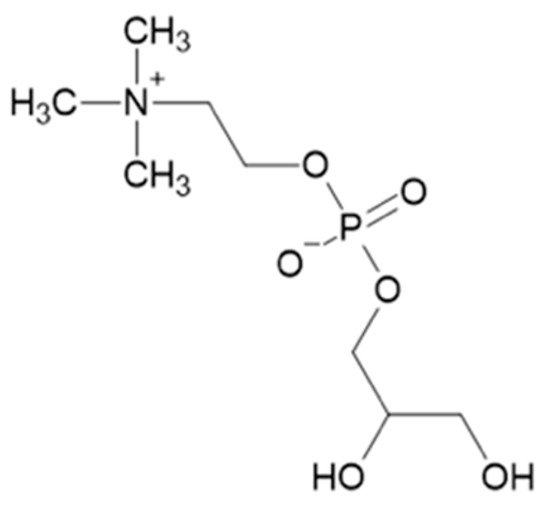
3. α-Glycerylphosphorycholine
4. Fusel Alcohols
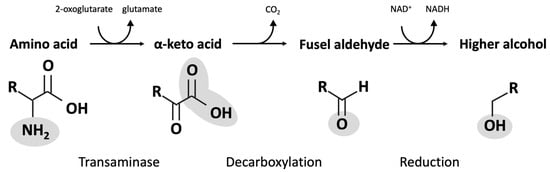
| Amino Acid | α-Keto Acid | Fusel Aldehyde | Fusel Alcohol |
|---|---|---|---|
| Isoleucine | α-Ketomethylvalerate | Methylvaleraldehyde | Active amyl alcohol |
| Leucine | α-Keotisocaproate | Isoamylaldehyde | Isoamyl alcohol |
| Methionine Phenylalanine Threonine |
α-Keto-γ-(methylthio)butyrate Phenylpyruvate 2-Ketobutyrate |
Methional Phenylethanal Propanal |
Methionol Phenylethanol Propanol |
| Tryptophan | 3-Indole pyruvate | 3-Indole acetaldehyde | Tryptophol |
| Tyrosine | p-Hydroxyphenylpyruvate | p-Hydroxyphenylacetaldehyde | p-Hydroxyphenylethanol or tyrosol |
| Valine | α-Ketoisovalerate | Isobutanal or isovaleraldehyde | Isobutanol |
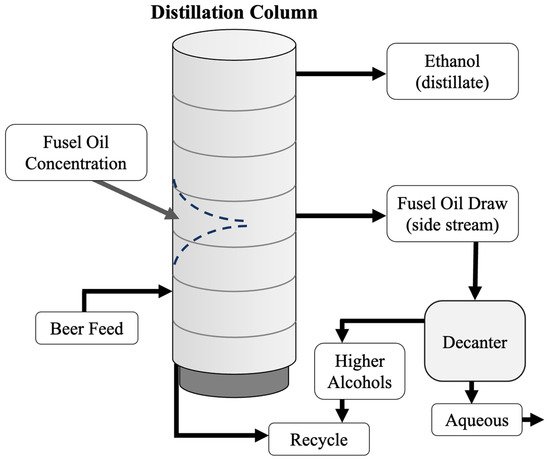
5. Two-Stage Fermentation of Thin Stillage

5.1. Organic Acids
5.2. Conversion of Glycerol to 1,3-Propanediol
5.3. Bacteriocins
6. Spent Yeast
References
- Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Production of bioethanol—A review of factors affecting ethanol yield. Fermentation 2021. Accepted.
- Tse, T.J.; Wiens, D.J.; Shen, J.; Beattie, A.D.; Reaney, M.J.T. Saccharomyces cerevisiae fermentation of 28 barley and 12 oat cultivars. Fermentation 2021, 7, 59.
- McCallum, B.D.; Depauw, R.M. A review of wheat cultivars grown in the Canadian prairies. Can. J. Plant Sci. 2008, 88, 649–677.
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2016, 2, 30.
- André, L.; Hemming, A.; Adler, L. Osmoregulation in Saccharomyces cerevisiae. Studies on the osmotic induction of glycerol production and glycerol-3-phosphate dehydrogenase (NAD+). FEBS Lett. 1991, 286, 13–17.
- Larsson, K.; Ansell, R.; Eriksson, P.; Adler, L. A gene encoding sn-glycerol 3-phosphate dehydrogenase (NAD+) complements an osmosensitive mutant of Saccharomyces cerevisiae. Mol. Microbiol. 1993, 10, 1101–1111.
- Ansell, R.; Granath, K.; Hohmann, S.; Thevelein, J.M.; Adler, L. The two isoenzymes for yeast NAD+-depedent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 hav distinct roles in osmoadaptation and redox regulation. EMBO. J. 1997, 16, 2179–2187.
- Gil, I.D.; Gómez, J.M.; Rodríguez, G. Control of an extractive distillation process to dehydrate ethanol using glycerol as entrainer. Comput. Chem. Eng. 2012, 39, 129–142.
- Hazelwood, L.A.; Daran, J.-M.; van Maris, A.J.A.; Pronk, J.T.; Dickinson, R.J. Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266.
- Sentheshanmuganathan, S. The mechanism of the formation of higher alcohols from amino acids by Saccharomyces cerevisiae. Biochem. J. 1960, 74, 568–576.
- Janssen, P.H. Propanol as an end product of threonine fermentation. Arch. Microbiol. 2004, 182, 482–486.
- Oyeneye, A.; Shen, J.; Shim, Y.Y.; Tse, T.J.; Reaney, M.J.T. Production of α—Glycerylphosphorylcholine and other compounds from wheat fermentation. ACS Omega 2020, 5, 12486–12494.
- Ratanapariyanuch, K.; Shin, Y.Y.; Emami, S.; Reaney, M.J.T. Production of protein concentrate and 1,3-propanediol by wheat-based thin stillage fermentation. J. Agric. Food. Chem. 2017, 65, 3858–3867.
- Tse, T.J.; Shen, J.; Shim, Y.Y.; Reaney, M.J.T. Changes in bacterial populations and their metabolism over 90 sequential cultures on wheat-based thin stillage. J. Agric. Food. Chem. 2020, 68, 4717–4729.
- Grand View Research. Ethanol Market Size, Share & Trends Analysis Report by Source (Second Generation, Grain-Based), by Purity (Denatured, Undenatured), by Application (Beverages, Fuel & Fuel Additives), and Segment Forecasts, 2020–2027; Grand View Research: San Francisco, CA, USA, 2020.
- Grand View Research. Acetic Acid Market Size, Share and Trends Analysis Report by Application (Vinyl Acetate Monomer, Purified Terephthalic Acid, Acetate Esters, Ethanol), by Region, and Segment Forecasts, 2020–2027; Grand View Research: San Francisco, CA, USA, 2020.
- Grand View Research. Succinic Acid Market Size, Share & Trends Analysis Report by Application, by Region (North America, Europe, Asia Pacific, Row), and Segment Forecasts, 2015–2022; Grand View Research: San Francisco, CA, USA, 2016.
- Grand View Research. Lactic Acid Market Size, Share and Trends Analysis Report by Raw Material (Sugarcane, Corn, Cassava), by Application (Pla, Food & Beverages), by Region, and Segment Forecasts, 2021–2028; Grand View Research: San Francisco, CA, USA, 2021.
- Grand View Research. Glycerol Market Size, Share & Trends Analysis Report by Source (Biodiesel, Fatty Acids, Fatty Alcohols, Soap), by Type (Crude, Refined) by End Use (Food & Beverage, Pharmaceutical), by Region, and Segment Forecasts, 2020–2027; Grand View Research: San Francisco, CA, USA, 2020.
- Market Watch. Nootropics Market Size Rising at CAGR of 12.5% during 2021-2028: Global Industry Brief Analysis of Top Countries Data, Trends and Drivers with Top Key Players. Available online: https://www.marketwatch.com/press-release/nootropics-market-size-rising-at-cagr-of-125-during-2021-2027-global-industry-brief-analysis-of-top-countries-data-trends-and-drivers-with-top-key-players-2021-07-22 (accessed on 4 October 2021).
- Market Watch. DDGS Market Size in 2021: 5.2% CAGR with Top Countries Data, Global Forecast to 2026 by Trends, Product Type, Future Growth, Leading Key Players, Demand Forecast and Revenue Analysis. 2021. Available online: https://www.marketwatch.com/press-release/ddgs-market-size-in-2021-52-cagr-with-top-countries-data-global-forecast-to-2026-by-trends-product-type-future-growth-leading-key-players-demand-forecast-and-revenue-analysis-updated-117-pages-report-2021-08-18 (accessed on 4 October 2021).
- Ham, G.A.; Stock, R.A.; Klopfenstein, T.J.; Larson, E.M.; Shain, D.H.; Hanke, H.E. Wet corn distillers byproducts compared with dried corn distillers’ grains with solubles as a source of protein and energy for ruminants. J. Anim. Sci. 1994, 72, 3246–3257.
- Wu, Y.V.; Sexson, K.R.; Lagoda, A.A. Protein-rich residue from wheat alcohol distillation: Fractionation and characterization. Cereal Chem. 1984, 61, 423–427.
- Kwiatkowski, J.R.; McAloon, A.J.; Taylor, F.; Johnston, D.B. Modeling the process and costs of fuel ethanol production by the corn dry-grind process. Ind. Crops Prod. 2006, 23, 288–296.
- Mustafa, A.F.; McKinnon, J.J.; Ingledew, M.W.; Christensen, D.A. The nutritive value for ruminants of thin stillage and distillers’ grains derived from wheat, rye, triticale and barley. J. Agric. Food Chem. 2000, 80, 607–613.
- Castellari, M.; Versari, A.; Fabiani, A.; Parpinello, G.P.; Galassi, S. Removal of ochratoxin A in red wines by means of adsorption treatments with commercial fining agents. J. Agric. Food Chem. 2001, 49, 3917–3921.
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. Water Treatment: Principles and Design, 2nd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2005.
- Chadwick, T.H.; Schroeder, E.D. Characterization and treatability of pomace stillage. J Water Pollut. Control Fed. 1973, 45, 1978–1984.
- Burke, D.A. Application of the AGF (Anoxic Gas Flotation) Process. Paper Presented at the 4th International Conference on Flotation in Water and Waste Water Treatment; Finnish Water and Wastewater Works Association: Helsinki, Finland, 2000.
- Macfarlane, A.; Prestidge, R.; Farid, M.; Chen, J. Dissolved air flotation: A novel approach to recovery of organosolv lignin. Chem. Eng. J. 2009, 148, 15–19.
- Arora, A.; Dien, B.S.; Belyea, R.L.; Singh, V.; Tumbleson, M.E.; Rausch, K.D. Nutrient recovery from the dry grind process using sequential micro and ultrafiltration of thin stillage. Bioresour. Technol. 2010, 101, 3859–3863.
- Bento, J.M.A.; Fleming, H.L. Membrane-Based Process for the Recovery of Lactic Acid and Glycerol from a “Corn Thin Stillage” Stream. U.S. Patent 5,250,182A, 14 January 1994.
- Tse, T.J.; Reaney, M.J.T. Enrichment and Utilization of Thin Stillage By-products. In Food Wastes and By-Products: Nutraceutical and Health Potential; Campos-Vega, R., Oomah, B.D., Vergara-Castañeda, H.A., Eds.; John Wiley & Son Ltd.: Hoboken, NJ, USA, 2019.
- Alotaibi, K.D.; Schoenau, J.J.; Hao, X. Fertilizer potential of thin stillage from wheat-based ethanol production. Bioenerg. Res. 2014, 7, 1421–1429.
- Ratanapariyanuch, K.; Tyler, R.T.; Shim, Y.Y.; Reaney, M.J.T. Biorefinery process for protein extraction from oriental mustard (Brassica juncea (L.) Czern.) using ethanol stillage. AMB Express. 2012, 2, 5.
- Lapišová, K.; Vlček, R.; Klozová, J.; Rychtera, M.; Melzoch, K. Separation techniques for distillery stillage treatment. Czech J. Food Sci. 2006, 24, 261–267.
- Scheimann, D.W. Method of Dewatering Grain Stillage Solids. U.S. Patent 7,566,469 B2, 14 May 2009.
- Ingledew, W.M.; Austin, G.D.; Kelsall, D.R.; Kluhspies, C. The alcohol industry: How has it changed and matured. In The Alcohol Textbook, 5th ed.; Ingledew, W.M., Kelsall, D.R., Austin, G.D., Kluhspies, C., Eds.; Nottingham University Press: Nottingham, UK, 2009.
- Bhadra, R.; Muthukumarappan, K.; Rosentrater, K.A.; Kannadhason, S. Drying kinetics of Distillers Wet Grains (DWG) under varying Condensed Distillers Solubles (CDS) and temperature levels. Cereal Chem. 2011, 88, 451–458.
- Rosentrater, K.A.; Ileleji, K.; Johnston, D.B. Manufacturing of Fuel Ethanol and Distillers Grains—Current and Evolving Processes. In Distillers Grains—Production, Properties, and Utilization; Liu, K.S., Rosentrater, K.A., Eds.; AOCS Publishing: Boca Raton, FL, USA, 2012.
- Singh, V.; Moreau, R.A.; Hicks, K.B.; Belyea, R.L.; Staff, C.H. Removal of Fiber from Distillers Dried Grains with Solubles (DDGS) to Increase Value. Trans. ASAE 2002, 45, 389–392.
- Villegas-Torres, M.F.; Ward, J.M.; Lye, G.J. The protein fraction from wheat-based dried dis-tiller’s grain with solubles (DDGS): Extraction and valorization. New Biotechnol. 2015, 32, 606–611.
- Kim, Y.; Hendrickson, R.; Mosier, N.S.; Ladisch, M.R.; Bals, B.; Balan, V.; Dale, B.E.; Dien, B.S.; Cotta, M.A. Effect of compositional variability of distillers’ grains on cellulosic ethanol production. Bioresour. Technol. 2010, 101, 5385–5393.
- Cantrell, D.F.; Winsness, D.J. Method of Recovering Oil from Thin Stillage. United. States Patent US 8,008,517 B2, 30 August 2011.
- Haas, M. Extraction and Use of DDGS Lipids for Biodiesel Production. In A Distillers Grains—Production, Properties, and Utilization; Liu, K.S., Rosentrater, K., Eds.; AOCS Publishing: Boca Raton, FL, USA, 2012.
- Sangiorgi, G.B.; Barbagallo, M.; Giordano, M.; Meli, M.; Panzarasa, R. Alpha-Glycerophosphocholine in the mental recovery of cerebral ischemic Attacks. Ann. N. Y. Acad. Sci. 1994, 717, 253–269.
- Choi, J.W.; Park, H.-Y.; Oh, M.S.; Yoo, H.H.; Lee, S.-H.; Ha, S.K. Neuroprotective effect of 6-paradol enriched ginger extract by fermentation using Schizosaccharomyces pombe. J. Funct. Foods 2017, 31, 304–310.
- Minnaar, P.; Jolly, N.; Paulsen, V.; Du Plessis, H.; Van Der Rijst, M. Schizosaccharomyces pombe and Saccharomyces cerevisiae yeasts in sequential fermentations: Effect on phenolic acids of fermented Kei-apple (Dovyalis caffra L.) juice. Int. J. Food Microbiol. 2017, 257, 232–237.
- Miljić, U.; Puškaš, V.; Vučurović, V.; Muzalevski, A. Fermentation Characteristics and Aromatic Profile of Plum Wines Produced with Indigenous Microbiota and Pure Cultures of Selected Yeast. J. Food Sci. 2017, 82, 1443–1450.
- Grand View Research. Brain Health Supplements Market Size, Share & Trends Analysis Report By Product (Natural Molecules, Herbal Extract), By Application (Memory Enhancement, Depression & Mood), By Region, And Segment Forecasts, 2021–2028. 2021. Available online: https://www.grandviewresearch.com/industry-analysis/brain-health-supplements-market (accessed on 8 October 2021).
- Hori, H.; Fujii, W.; Hatanaka, Y.; Suwa, Y. Effects of Fusel Oil on Animal Hangover Models. Alcohol. Clin. Exp. Res. 2003, 27, 37S–41S.
- Ehrlich, F. Über die Bedingungen der Fuselölbildung und über ihren Zusammenhang mit dem Eiweißaufbau der Hefe. Eur. J. Inorg. Chem. 1907, 40, 1027–1047.
- Pires, E.J.; Teixeira, J.; Brányik, T.; Vicente, A. Yeast: The soul of beer’s aroma—a review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949.
- Ferreira, M.C.; Meirelles, A.J.A.; Batista, E.A.C. Study of the Fusel alcohol Distillation Process. Ind. Eng. Chem. Res. 2013, 52, 2336–2351.
- Mendoza-Pedroza, J.D.J.; Sánchez-Ramírez, E.; Segovia-Hernández, J.G.; Hernández, S.; Orjuela, A. Recovery of alcohol industry wastes: Revaluation of fusel oil through intensified processes. Chem. Eng. Process. Process. Intensif. 2021, 163, 108329.
- Smit, B.A.; Engels, W.J.; Smit, G. Branched chain aldehydes: Production and breakdown pathways and relevance for flavour in foods. Appl. Microbiol. Biotechnol. 2009, 81, 987–999.
- Dos Santos, C.M.E.; Pietrowski, G.D.A.M.; Braga, C.; Rossi, M.J.; Ninow, J.; Dos Santos, T.P.M.; Wosiacki, G.; Jorge, R.M.M.; Nogueira, A. Apple Aminoacid Profile and Yeast Strains in the Formation of Fusel Alcohols and Esters in Cider Production. J. Food Sci. 2015, 80, 1170.
- Schoondermark-Stolk, S.A.; Tabernero, M.; Chapman, J.; Ter Schure, E.G.; Verrips, C.T.; Verkleij, A.J.; Boonstra, J. Bat2p is essential in Saccharomyces cerevisiae for fusel alcohol production on the non-fermentable carbon source ethanol. FEMS Yeast Res. 2005, 5, 757–766.
- Eden, A.; Van Nedervelde, L.; Drukker, M.; Benvenisty, N.; Debourg, A. Involvement of branched-chain amino acid aminotransferases in the production of fusel alcohols during fermentation in yeast. Appl. Microbiol. Biotechnol. 2001, 55, 296–300.
- Kang, T.S.; Korber, D.R.; Tanaka, T. Metabolic engineering of a glycerol-oxidative pathway in Lactobacillus panis PM1 for utilization of bioethanol thin stillage: Potential to produce platform chemicals from glycerol. Appl. Environ. Microbiol. 2014, 80, 7631–7639.
- Ratanapariyanuch, K.; Shim, Y.Y.; Wiens, D.J.; Reaney, M.J.T. Grain Thin Stillage Protein Utilization: A Review. J. Am. Oil Chem. Soc. 2018, 95, 933–942.
- Pedersen, C.; Jonsson, H.; Lindberg, J.E.; Roos, S. Microbiological Characterization of Wet Wheat Distillers’ Grain, with Focus on Isolation of Lactobacilli with Potential as Probiotics. Appl. Environ. Microbiol. 2004, 70, 1522–1527.
- Liu, L.; Redden, H.; Alper, H.S. Frontiers of yeast metabolic engineering: Diversifying beyond ethanol and Saccharomyces. Curr. Opin. Biotechnol. 2013, 24, 1023–1030.
- Yang, L.; Lübeck, M.; Lübeck, P.S. Aspergillus as a versatile cell factory for organic acid production. Fungal Biol. Rev. 2017, 31, 33–49.
- Liaud, N.; Giniés, C.; Navarro, D.; Fabre, N.; Crapart, S.; Gimbert, I.H.-; Levasseur, A.; Raouche, S.; Sigoillot, J.-C. Exploring fungal biodiversity: Organic acid production by 66 strains of filamentous fungi. Fungal Biol. Biotechnol. 2014, 1, 1–10.
- Ahn, J.H.; Jang, Y.-S.; Lee, S.Y. Production of succinic acid by metabolically engineering microorganisms. Curr. Opin. Biotechnol. 2016, 42, 54–66.
- Raab, A.M.; Lang, C. Oxidative versus reductive succinic acid production in the yeast saccharomyces cerevisiae. Bioeng. Bugs 2011, 2, 120–123.
- Ferone, M.; Raganati, F.; Ercole, A.; Olivieri, G.; Salatino, P.; Marzochella, A. Continuoous succinic acid fermentation by Actinobacillus succinogenes in a packed-bed biofilm reactor. Biotechnol. Biofuels. 2018, 11, 138.
- Abbott, D.A.; Zelle, R.; Pronk, J.; Van Maris, A.J.A. Metabolic engineering of Saccharomyces cerevisiae  for production of carboxylic acids: Current status and challenges. FEMS Yeast Res. 2009, 9, 1123–1136.
- Pielech-Przybylska, K.; Balcerek, M.; Ciepielowski, G.; Pacholczyk-Sienicka, B.; Albrecht, Ł.; Dziekońska-Kubczak, U.; Bonikowski, R.; Patelski, P. Effect of Co-Inoculation with Saccharomyces cerevisiae and Lactic Acid Bacteria on the Content of Propan-2-ol, Acetaldehyde and Weak Acids in Fermented Distillery Mashes. Int. J. Mol. Sci. 2019, 20, 1659.
- Sousa, M.J.; Ludovico, P.; Rodrigues, F.; Leão, C.; Côrte-Real, M. Stress and cell death in yeast induced by acetic acid. In Cell Metabolism—Cell Homeostasis and Stress Response; Bubulya, P., Ed.; InTech: Rijeka, Croatia, 2012.
- Kaur, G.; Srivastava, A.; Chand, S. Advances in biotechnological production of 1,3-propanediol. Biochem. Eng. J. 2012, 64, 106–118.
- Kraus, G.A. Synthetic Methods for the Preparation of 1,3-Propanediol. CLEAN—Soil Air Water 2008, 36, 648–651.
- Klaenhammer, T.R. Bacteriocins of lactic acid bacteria. Biochimie 1988, 70, 337–349.
- Riley, M.A. Molecular mechanisms of bacteriocin evolution. Annu. Rev. Genet. 1998, 32, 255–278.
- De Vuyst, L.; Vandamme, E.J. Antimicrobial Potential of Lactic Acid Bacteria. In Bacteriocins of Lactic Acid Bacteria; Springer International Publishing: Berlin/Heidelberg, Germany, 1994; pp. 91–142.
- Elayaraja, S.; Annamalai, N.; Mayavu, P.; Balasubramanian, T. Production, purification and characterization of bacteriocin from Lactobacillus murinus AU06 and its broad antibacterial spectrum. Asian Pac. J. Trop. Biomed. 2014, 4, S305–S311.
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129.
- Oscáriz, J.C.; Pisabarro, A.G. Classification and mode of action of membrane active bacteriocins produced by gram-positive bacteria. Int. Microbiol. 2001, 4, 13–19.
- Gänzle, M.G. Reutericyclin: Biological activity, mode of action, and potential applications. Appl. Microbiol. Biotechnol. 2004, 64, 326–332.
- Tagg, J.R.; Dajani, A.S.; Wannamaker, L.W. Bacteriocins of Gram-positive bacteria. Bacteriol. Rev. 1976, 40, 722–756.
- Héchard, Y.; Sahl, H.-G. Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie 2002, 84, 545–557.
- Moll, G.N.; Konings, W.N.; Driessen, A.J.M. Bacteriocins: Mechanism of membrane insertion and pore formation. Lact. Acid Bact. Genet. Metab. Appl. 1999, 76, 185–198.
- Parada, J.L.; Caron, C.R.; Medeiros, A.B.P.; Soccol, C.R. Bacteriocins from lactic acid bacteria: Purification, properties and use as biopreservatives. Braz. Arch. Biol. Technol. 2007, 50, 521–542.
- Todorov, S.D. Bacteriocins from Lactobacillus plantarum production, genetic organization and mode of action: Produção, organização genética e modo de ação. Braz. J. Microbiol. 2009, 40, 209–221.
- Vossen, J.M.; Herwijnen, M.H.; Leer, R.J.; Brink, B.T.; Pouwels, P.H.; Huis in `t Veld, J.H.J. Production of acidocin B, a bacteriocin of Lactobacillus acidophilus M46 is a plasmid-encoded trait: Plasmid curing, genetic marking by in vivo plasmid integration, and gene transfer. FEMS Microbiol. Lett. 1994, 116, 333–340.
- Vescovo, M.; Morelli, L.; Bottazzi, V.; Gasson, M.J. Conjugal Transfer of Broad-Host-Range Plasmid pAMbeta1 into Enteric Species of Lactic Acid Bacteria. Appl. Environ. Microbiol. 1983, 46, 753–755.
- Ceapa, C.; Davids, M.; Ritari, J.; Lambert, J.; Wels, M.; Douillard, F.P.; Smokvina, T.; de Vos, W.M.; Knol, J.; Kleerebezem, M. The variable regions of Lactobacillus rhamnosus genomes reveal the dynamic evolution of metabolic and host-adaptation repertoires. Genome Biol. Evol. 2016, 8, 1889–1905.
- Baugher, J.L.; Durmaz, E.; Klaenhammer, T.R. Spontaneously Induced Prophages in Lactobacillus gasseri Contribute to Horizontal Gene Transfer. Appl. Environ. Microbiol. 2014, 80, 3508–3517.
- Mercanti, D.J.; Rousseau, G.M.; Capra, M.L.; Quiberoni, A.; Tremblay, D.M.; Labrie, S.; Moineau, S. Genomic Diversity of Phages Infecting Probiotic Strains of Lactobacillus paracasei. Appl. Environ. Microbiol. 2016, 82, 95–105.
- Tannock, G.W.; Luchansky, J.B.; Miller, L.; Connell, H.; Thode-Andersen, S.; Mercer, A.; Klaenhammer, T.R. Molecular Characterization of a Plasmid-Borne (pGT633) Erythromycin Resistance Determinant (ermGT) from Lactobacillus reuteri 100-63. Plasmid 1994, 31, 60–71.
- Egervärn, M.; Roos, S.; and Lindmark, H. Identification and characterization of antibiotic resistance genes in Lactobacillus reuteri and Lactobacillus plantarum. J. Appl. Microbiol. 2009, 107, 1658–1668.
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s Spent Yeast (BSY), an Underutilized Brewing By-Product. Fermentation 2020, 6, 123.
- Vieira, E.F.; Carvalho, J.; Pinto, E.; Cunha, S.; Almeida, A.; Ferreira, I.M. Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J. Food Compos. Anal. 2016, 52, 44–51.
- Jacob, F.F.; Striegel, L.; Rychlik, M.; Hutzler, M.; Methner, F.-J. Yeast extract production using spent yeast from beer manufacture: Influence of industrially applicable disruption methods on selected substance groups with biotechnological relevance. Eur. Food Res. Technol. 2019, 245, 1169–1182.
- Pinto, L.; Lopes, M.; Filho, C.C.; Alves, L.; Benevides, C. Determinação do valor nutritivo de derivados de levedura de cervejaria (Saccharomyces spp.). Rev. Bras. de Prod. Agroind. 2013, 15, 7–17.
- Pinto, M.; Coelho, E.; Nunes, A.; Brandão, T.; Coimbra, M.A. Valuation of brewers spent yeast polysaccharides: A structural characterization approach. Carbohydr. Polym. 2015, 116, 215–222.
- Liu, C.-H.; Hwang, C.-F.; Liao, C.-C. Medium optimization for glutathione production by Saccharomyces cerevisiae. Process. Biochem. 1999, 34, 17–23.
- Bayarjargal, M.; Munkhbat, E.; Ariunsaikhan, T.; Odonchimeg, M.; Uurzaikh, T.; Gan-Erdene, T.; Regdel, D. Utilization of spent brewer’s yeast Saccharomyces cerevisiae for the production of yeast enzymatic hydrolysate. Mong. J. Chem. 2014, 12, 88–91.




