Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Domenico Baldini | + 3165 word(s) | 3165 | 2021-12-22 03:13:14 | | | |
| 2 | Catherine Yang | Meta information modification | 3165 | 2022-01-10 11:09:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Baldini, D. Sperm Selection for Intracytoplasmic Sperm Injection. Encyclopedia. Available online: https://encyclopedia.pub/entry/17966 (accessed on 07 February 2026).
Baldini D. Sperm Selection for Intracytoplasmic Sperm Injection. Encyclopedia. Available at: https://encyclopedia.pub/entry/17966. Accessed February 07, 2026.
Baldini, Domenico. "Sperm Selection for Intracytoplasmic Sperm Injection" Encyclopedia, https://encyclopedia.pub/entry/17966 (accessed February 07, 2026).
Baldini, D. (2022, January 10). Sperm Selection for Intracytoplasmic Sperm Injection. In Encyclopedia. https://encyclopedia.pub/entry/17966
Baldini, Domenico. "Sperm Selection for Intracytoplasmic Sperm Injection." Encyclopedia. Web. 10 January, 2022.
Copy Citation
Intracytoplasmic sperm injection (ICSI) is the method that has definitely revolutionized the field of ART since normal fertilization and ongoing pregnancies can be achieved even with low quality sperm samples and affected spermatozoa. By injecting a single sperm cell in the oocyte, the technique bypasses several biological barriers that naturally select the gametes to achieve optimal embryonic and fetal development.
assisted reproductive technology (ART)
in vitro fertilization (IVF)
ICSI
1. Natural Sperm Selection
In nature, the process that leads to the selection of the best sperm cells, capable of fertilizing the oocyte in the female genital tract, is very selective and it involves high morphologic qualities and dynamic features. Leaving the seminal plasma in the vagina, sperm cells perform the first step that leads to this process.
Only a small portion of the sperm cells that swim from the uterus to the oviduct can be collected in niches, where cells interact with unknown receptors to form a sperm reserve, as in some animal species the sperm cells can stay in the fallopian tubes even for months before ovulation. However, in the human species, the fertilization window is reduced, and the sperm cells can survive no longer than 5/6 days [1]. This process allows the sperm to reach complete capacitation, which is based on plasma membrane cholesterol ultrastructure changes; it leads to an increase in concentration of intracellular ions, such as Ca2+, that switch the motility patterns of tails to hyper-activation. Moreover, phosphorylation on tyrosine residues determines the preparation for the acrosomal reaction event [2]; subsequent interactions with molecules, such as secreted protein or hormones, from the female reproductive tract, modulate the swim, by a chemotactic and thermotactic effect, towards the oocyte.
All current methodologies improve sperm cells quality, but none have been associated with a significant increase in clinical results [3]. Methods for sperm cells’ selection are categorized into classic techniques based on sperm motility or density, and advanced methods that rely on membrane surface charge, high-resolution morphology, and nuclear or membrane integrity. Nowadays, the most used techniques in the ART lab are SU and DGC [4]. Here below the Table 1 represent a summary of all the sperm cell preparation techniques with advantages and disadvantages of each of them.
Table 1. Advantages and disadvantages of sperm cells’ selection techniques.
| Procedures | Advantages | Disadvantages |
|---|---|---|
| Swim-up | ||
| Centrifugation on density gradient | ||
| HOST | ||
| Polarization microscopy |
|
|
| LAISS | ||
| MACS |
|
|
| PICSI and selection with hyaluronic acid |
|
|
| Zeta potential | ||
| IMSI |
|
|
| Microfluidic separation |
|
|
| Horizontal Sperm migration |
2. Advanced Methods
2.1. Selection Methods for Sperm Cells with Reduced Motility
When the sperm sample is retrieved by testicular aspiration (TESE) [50] or epididymal aspiration (MESA) [51], the spermatozoa might appear immotile due to the lack of complete maturation that takes place in the final tract of the epididymis [52].
It is important to underline that in these cases, the SU and DGC techniques, that exploit dynamic characteristics for separation, are inadequate.
An alternative selection technique is the hypo-osmotic swelling test (HOST) [14]. This method (Figure 1) assumes that the tails of viable spermatozoa swell and bend if they are introduced into a hypoosmotic environment, due to the activity of the osmo-sensitive calcium membrane channels [53][54]. HOST can be used to estimate the percentage of integrity in chromatin.
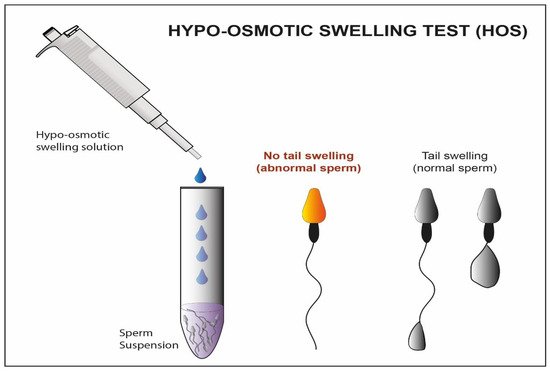
Figure 1. Schematic representation Hypo-osmotic swelling Test. In this figure, we observe the difference between the abnormal sperm with not swelled tail and the normal sperm with swelled tail after treatment with a hypo-osmotic solution.
Using polarized light implemented in the microscope (Figure 2) is another possible strategy for the selection of immotile sperm cells [19]. Some research teams have proposed its use to verify the birefringence of sperm cell head as an index of suitability. As a matter of fact, the sub-acrosomal protein filaments extend themselves longitudinally, giving a typical pattern of birefringence to the sperm cell head [20]. In some published studies, the selection of non-motile spermatozoa with birefringent heads resulted in an increase in clinical pregnancy and implantation rate (58% vs. 9% and 42% vs. 12%), compared to a control group with immotile spermatozoa (where polarized light was not used) [55], and an increased implantation rate when the HOST technique was used instead as selection method (45% vs. 11%) [56].
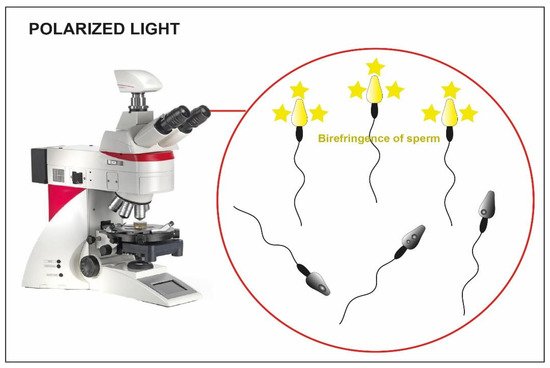
Figure 2. Microscope implemented with polarized light. The birefringence of the heads is clear in the viable sperm (yellow heads) compared to the not viable one where the birefringence is absent (dark heads).
Unfortunately, even this technique presents some disadvantages: the polarized light implemented in the microscope is expensive and there is a lack of data regarding the integrity of the sperm DNA. Furthermore, the success of the latter is based largely on the experience of the operator.
A group of researchers has shown another potential method to recover immotile but suitable sperms for ICSI. It exploits the chemical inducers of motility (Figure 3), that belong to the class of phosphodiesterase inhibitors, such as pentoxifylline (PTF), dimethylxanthines and papaverines [57], allowing the motility reactivation of spermatozoa recovered from the testicular and epididymal level [58].
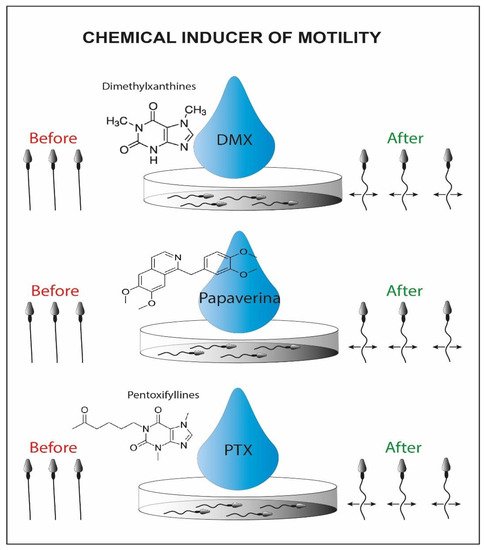
Figure 3. Schematic representation of chemical inducers of motility. On the left we can recognize the initial immotile sperms and on the right side the viable motile sperms activated by the chemical inducer.
Alternatively, motility can be further reactivated by using a laser incorporated in the magnification system that is shot at the immotile sperm cell tail (LAISS laser-assisted immotile sperm selection) (Figure 4) [21]. Laser irradiation causes the release in cytosol of second messengers, such as Ca2+ or ROS, and an increase in the synthesis of ATP, which could generate a slight movement of the tail [59]. The sperm cell is then considered viable when its tail is coiled after the laser shot [22]. On the other hand, some authors claim that high laser doses cause an excess of potentially toxic ROS [25]. Furthermore, more Ca2+ influx induces the hyperactivity of Ca2+-ATPase calcium channels and exhausts the ATP reserves of the cell. This process could lead to depletion of cell channels activity, correlated with an increase in internal osmotic pressure causing the swelling of the sperm cell and subsequently the rupture of its plasma membrane [26]. Contrary, other authors suggest that this technique does not damage the spermatic membrane and does not affect the percentage of fragmentation of the genetic material [60].
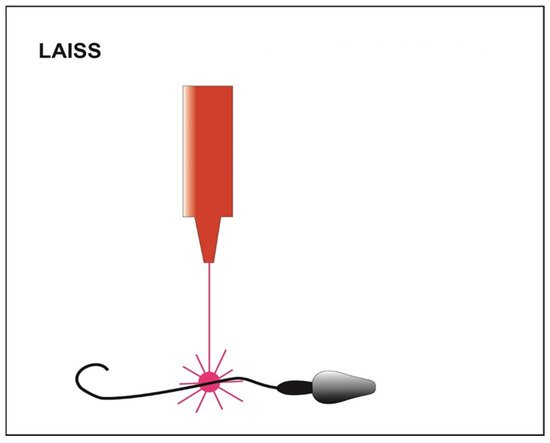
Figure 4. Schematic representation of LAISS (laser-assisted immotile sperm selection). The laser irradiation generates a slight movement of the tail in those viable sperms initially immotile.
It has also been demonstrated that the use of ATP/MgSO4 generates an increase in motility in testicular seminal samples, generating a weak contraction of the flagellum (Figure 5). The results of the study have shown that using ATP as a solute, the induced motility increases significantly, and viable sperm cells can be captured and used for injection through ICSI [61].
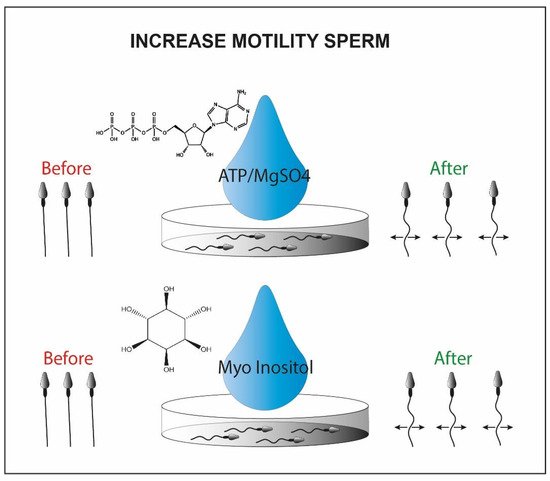
Figure 5. Sperm motility enhanced by ATP/MgSo4 or myo-inositol. Briefly in the picture we observe the immotile sperm before the treatment with the chemical inducer (left side) and the motile sperm cells after the chemical exposure (right side).
Alternatively, the myoinositol can be used to isolate viable sperm cells for ICSI. It represents the most abundant stereoisomer of the inositol class modulating the intracellular concentration of Ca2+ [62]. This is synthesized in two steps by two enzymes, myo-1-phosphate synthase and myo-monophosphatase-1, located in high concentration in the testicular mesenchymal tissue [63][64]. By incubating sperm cells frozen and then thawed from oligoasthenospermic patients with myoinositol, some research groups were able to recover a portion of sperm cells with significantly increased motility [65].
2.2. Sperm Cells Selection by Membrane Characteristics
The outer sperm membrane is critical for their functionality since it is firstly involved in many aspects of the fertilization such as capacitation, oocyte binding and acrosome reaction [66][67]. In order to select high-quality sperm cells, methods that gain benefits from the characteristics of their membrane have been studied.
A further method that takes advantage of the membrane characteristics is the magnetic activated cell sorting (MACS). This methodology allows the selection of the non-apoptotic portion from a sample of interest [68]. It involves the use of magnetic microspheres conjugated to Annexin V (AV-MACS) (Figure 6) [69], that have a high affinity for phosphatidyl-serine. The latter is normally exposed on the outer side of the membrane when the sperm cells are in an apoptotic state [70]. The seminal sample of interest passes through a column containing microspheres, to which the annexin has adhered. The non-viable spermatozoa remain trapped inside the column, while the viable fraction is eluted, improving the vitality characteristics of the starting sample [27][28].
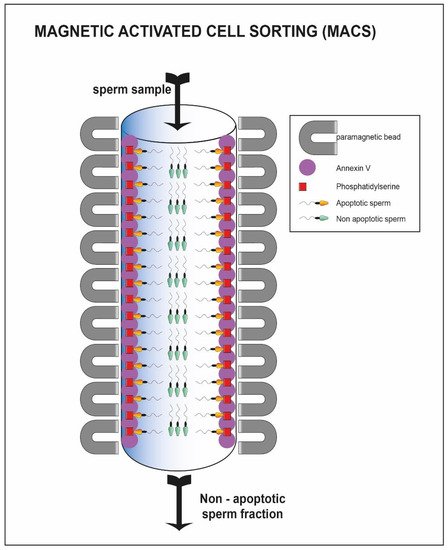
Figure 6. Sperm cell passing through column with annexin V. The sperm cells able to pass through the column represent the viable one (green heads) while the apoptotic fraction remain trapped inside the column (yellow heads).
Hyaluronic acid is the main component of the extracellular matrix surrounding the cumulus-oocyte complex [71]. Only the sperm cells that have successfully completed spermatogenesis and maturation are able to show the polysaccharide-binding receptors on their outer membrane [32][33]. Moreover, they are usually characterized by a normal morphology and a low percentage of fragmentation of the nuclear material [34]. Exploiting these characteristics, a methodology for sperm cell selection has been devised where spermatozoa are incubated in plates or in media containing hyaluronic acid; only the sperm cells able to bind the molecule are then used to perform a modified version of ICSI: physiological intracytoplasmic sperm injection (PICSI) [72][73]. The selection should result in an increased fertilization rate, but in this case, the clinical data are conflicting. Some studies suggest that both the fertilization rate and the percentage of top-quality embryos benefit from the technique (92 vs. 86; 36 vs. 24%) [74], but other research groups do not confirm these improvements [35]. Studies show that a negative charge is present on the sperm cell membrane [75]. The Zeta method (Figure 7) uses this feature to separate sperm cells containing the Y chromosome from those containing the X chromosome [36]. Two distinct research groups have developed two different methodologies, one using a positively charged centrifuge tube [40], the other using migration in an electrophoretic field [41], which allow the collection of live spermatozoa with normal morphology and a high percentage of the integrity of the genetic material [37][38][76][77].
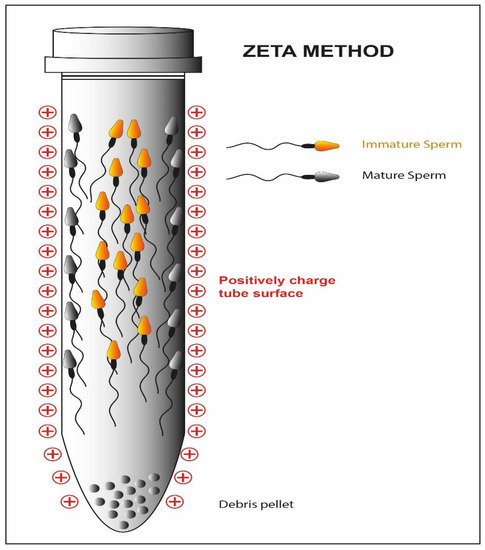
Figure 7. Schematic representation of the Zeta method. The high negative charge of the mature sperm cell membrane reacts with the positive charge of the tube while the immature fraction is detached form the tube.
2.3. Selection Based on Morphology–IMSI
The analysis of semen quality has for a long time been associated with the morphological evaluation of spermatozoa. The introduction of digital microscopy has made its possible to analyze the ultrastructural characteristics of motile spermatozoa (MSOME; motile sperm organelle morphology examination) [78]. The integration of the MSOME into the ICSI method allows a high-magnificence micro-injection (IMSI) (Figure 8) [79]. The selection of motile spermatozoa with few vacuoles and normal nuclear morphology is possible through a 6000× magnification system integrated with the micromanipulation system [80].
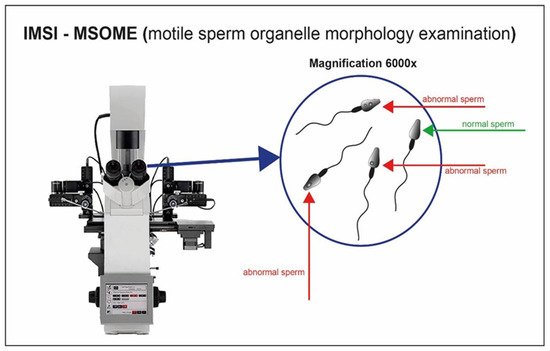
Figure 8. Sperm cell visualization with magnification system 6000×. Sperm cells with appropriate morphology are well selected through the high magnification.
2.4. Dynamic Selection of Spermatozoa
The three main mechanisms that control the movements of spermatozoa within the oviducts, rheotaxis, chemotaxis and thermotaxis, have been exploited as methods for sperm cell selection, to improve the ART outcomes:
-
Human spermatozoa orient their motion, by rheotaxis, against the fluid stream directed towards them [81]. Rheotaxis is defined as the tendency of certain living beings to move in response to the mechanical stimulus of a current of water. Some studies have confirmed the presence of a flow-directed towards the uterus in the oviductal region, capable of increasing its intensity after intercourse, which is able to attract the sperm cells [82]. In the technique developed by Nagata et al. [83], using bull sperm as a mammalian model, a flow is created through a series of microchannels towards a well where the sperm sample is delivered. In response to the flow, the spermatozoa swim towards it passing through the microchannels and finally reaching a receptive well where they can be collected for ART applications. Another study was conducted on normospermic patients [84] to select spermatozoa using rheotaxis: compared to an untreated sample and a sample subjected to density gradient centrifugation, the recovered spermatozoa showed higher chromatin compactness (99% vs. 71% vs. 83%).
-
Progesterone plays the main role as a chemo-attractor for the navigation of spermatozoa through the environment surrounding the cumulus-oocyte complex [85]. Only capacitated spermatozoa possess the receptors to recognize and bind this type of molecule [85]. Some sperm cell selection techniques exploit this feature to distinguish capacitated spermatozoa from non-capacitated ones. Several studies have been conducted on different types of sperm populations, using a device where a progesterone concentration gradient is constituted (sperm selection assay) [86]. In this method, two wells are connected by a 2 mm length per 2.5 mm diameter tube. One of the wells is filled with a media containing the chemoattractant molecule in solution (progesterone for instance) and the other well is filled with the human sperm sample. The chemoattractant diffuses through the tube generating a gradient and the spermatozoa respond by moving towards the higher concentration and accumulating in the initial well free of cells where they can be used for ART applications. The recovered spermatozoa from this technique exhibit better morphology, less DNA fragmentation and a reduced rate of apoptosis as compared to those selected with density gradient centrifugation [87]. Although the results are also promising in this case, further studies need to be conducted to confirm the improvements in the clinical field.
-
The spermatozoa can direct their motion according to the variation in temperature, moving from cold areas to warmer areas [88]. Several studies show that this mechanism underlies the movement of sperm cells from the fallopian tubes to the ampulla [89][90]. Even in this case, however, only the capacitated spermatozoa can respond to the temperature gradient, making this motion a mechanism for the selection of sperm cells with better fertilizing characteristics. The method of sperm selection by thermotaxis was developed by Pérez-Cerezales et al. [91].
2.5. Microfluidics Applied to Sperm Selection
Technologies related to microfluidics are rapidly growing within ART laboratories. Among the first experiments with this methodology, Smith and Takayama [46] have published a series of articles demonstrating the efficiency of the method for the selection of high-quality spermatozoa.
By controlling fluid dynamics (Figure 9), within millimeter diameter capillaries, it is possible to mimic the physiological conditions of pH and temperature of the female genital tract [92]. Hence, we could potentially select spermatozoa with increased motility through flows [93], chemical gradients [94] or electrophoretic fields [95]. The method of Smith and Takayama [46] uses two parallel laminar flow channels. While the motile spermatozoa can move through the flows and be eluted separately, the debris and immotile cells are passively transported from the entrance to the exit of the capillary canal.
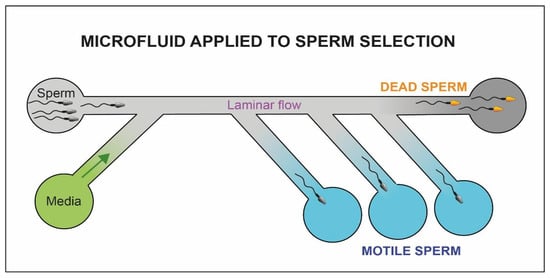
Figure 9. Schematic representation of the microfluidic system. The motile fraction of sperm cells is able to swim through the flow and be collected in separate chambers (blue chambers) while the immotile sperm cells (yellow heads) and debris reach the exit of the microfluidic system (dark chamber).
The application of optical systems to microfluidics could further improve the selection system [96]. Raman spectroscopy allows the discernment of sperm cells with high nuclear integrity from sperm cells with fragmented DNA [97]. By coupling the three-dimensional imaging to the selected channels, it is possible to accurately identify the different types of flagellar movement using a digital sensor, consisting of a semi-conductive material, which can virtually reconstruct the cell volume by recording the differences in the types of interference between a reference light wave and a scatter from the sample [98]. Using this method, De Wagenaar B. et al., were able to trace the profile of the flagellar beat of hyper-activated spermatozoa [99].
2.6. Horizontal Sperm Migration
During ICSI, an adequate number of spermatozoa reaches the distal edge of the furthest drop; some of these can be recovered from the injection needle, to be moved to the PVP and carefully selected for fertilization (Figure 10).
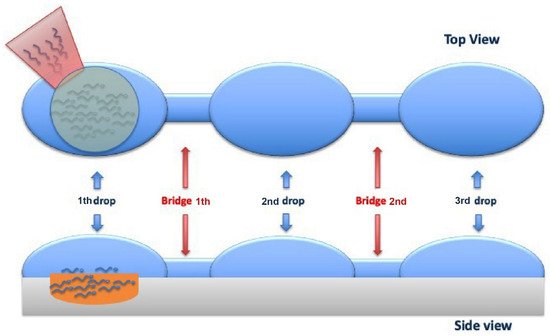
Figure 10. Top and Side viewing of the sperm cells horizontal migration from the first drop (where the cells are added) to the third drop (where the sperm cells are aspirated) through 2 bridges that link them.
The technique allows the recovery of spermatozoa with high motility, normal morphology and minimal damage to the DNA, using a fast, safe, and economical procedure. Comparing the clinical results obtained from this new selection method with the classic swim-up technique, no significant differences were found in terms of fertilization and implantation rate. On the other hand, segmentation and blastocyst rates are higher in the horizontal swim-up, suggesting that this process that generates a lower quantity of harmful oxygen reactants is correlated with better seminal quality and a lower DNA fragmentation.
References
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Update 2006, 12, 23–27.
- Puga Molina, L.C.; Luque, G.M.; Balestrini, P.A.; Marín-Briggiler, C.I.; Romarowski, A.; Buffone, M.G. Molecular Basis of Human Sperm Capacitation. Front. Cell Dev. Biol. 2018, 6, 72.
- ESHRE Guideline Group on Good Practice in IVF Labs; De los Santos, M.J.; Apter, S.; Coticchio, G.; Debrock, S.; Lundin, K.; Vermeulen, N. Revised guidelines for good practice in IVF laboratories. Hum. Reprod. 2015, 31, 685–686.
- Vaughan, D.A.; Sakkas, D. Sperm selection methods in the 21st century. Biol. Reprod. 2019, 101, 1076–1082.
- Lopata, A.; Patullo, M.J.; Chang, A.; James, B. A method collecting motile spermatozoa from human semen. Fertil. Steril. 1976, 27, 677.
- Bui, A.D.; Sharma, R.; Henkel, R.; Agarwal, A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia 2018, 50, e13012.
- Ghumman, S.; Adiga, S.K.; Upadhya, D.; Kalthur, G.; Jayaraman, V.; Rao, S.B.; Kumar, P. Combination of swim-up and density gradient separation methods effectively eliminate DNA damaged sperm. J. Turk. Ger. Gynecol. Assoc. 2011, 12, 148–152.
- Jayaraman, V.; Upadhya, D.; Narayan, P.K.; Adiga, S.K. Sperm processing by swim-up and density gradient is effective in elimination of sperm with DNA damage. J. Assist. Reprod. Genet. 2012, 29, 557–563.
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021.
- Henkel, R.R.; Schill, W.B. Sperm preparation for ART. Reprod. Biol. Endocrinol. 2003, 1, 108.
- Harrison, R.A. A highly efficient method for washing mammalian spermatozoa. J. Reprod. Fertil. 1976, 48, 347–353.
- Lee, D.; Jee, B.C. Evaluation of normal morphology, DNA fragmentation, and hyaluronic acid binding ability of human spermatozoa after using four different commercial media for density gradient centrifugation. Clin. Exp. Reprod. Med. 2019, 46, 8–13.
- Strehler, E.; Baccetti, B.; Sterzik, K.; Capitani, S.; Collodel, G.; De Santo, M.; Gambera, L.; Piomboni, P. Detrimental effects of polyvinylpyrrolidone on the ultrastructure of spermatozoa (Notulae seminologicae 13). Hum. Reprod. 1998, 13, 120–123.
- Jeyendran, R.S.; Van der Ven, H.H.; Perez-Pelaez, M.; Crabo, B.G.; Zaneveld, L.J. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J. Reprod. Fertil. 1984, 70, 219–228.
- Bassiri, F.; Tavalaee, M.; Shiravi, A.H.; Mansouri, S.; Nasr-Esfahani, M.H. Is there an association between HOST grades and sperm quality? Hum Reprod. 2012, 27, 2277–2284.
- Bloch, A.; Rogers, E.J.; Nicolas, C.; Martin-Denavit, T.; Monteiro, M.; Thomas, D.; Morel, H.; Lévy, R.; Siffroi, J.P.; Dupont, C.; et al. Detailed cell-level analysis of sperm nuclear quality among the different hypo-osmotic swelling test (HOST) classes. J. Assist. Reprod. Genet. 2021, 38, 2491–2499.
- Casper, R.F.; Meriano, J.S.; Jarvi, K.A.; Cowan, L.; Lucato, M.L. The hypo-osmotic swelling test for selection of viable sperm for intracytoplasmic injection in men with complete asthenozoospermia. Fertil. Steril. 1996, 65, 972–976.
- Tsai, Y.L.; Liu, J.; Garcia, J.E.; Katz, E.; Compton, G.; Baramki, T.A. Establishment of optimal hypo-osmotic swelling test by examining single spermatozoa from different hypo-osmotic solutions. Hum. Reprod. 1997, 12, 11–113.
- Vermey, B.G.; Chapman, M.G.; Cooke, S.; Kilani, S. The relationship between sperm head retardance using polarized light microscopy and clinical outcomes. Reprod. Biomed. Online 2015, 30, 67–73.
- Baccetti, B. Microscopical advances in assisted reproduction. J. Submicrosc. Cytol. Pathol. 2004, 36, 333–339.
- Aktan, T.M.; Montag, M.; Duman, S.; Gorkemli, H.; Rink, K.; Yurdakul, T. Use of a laser to detect viable but immotile spermatozoa. Andrologia 2004, 36, 366–369.
- Birowo, P.; Tendi, W.; Rasyid, N.; Turek, P.J.; Sini, I.R.; Rizal, M. Successful Targeted Testicular Sperm Extraction Using Microsurgical Technique (microTESE) Following Fine Needle Aspiration (FNA) Mapping in a Non-Obstructive Azoospermia (NOA) Patient: A Case Report. J. Reprod. Infertil. 2021, 22, 65–69.
- Gerber, P.A.; Kruse, R.; Hirchenhain, J.; Krüssel, J.S.; Neumann, N.J. Pregnancy after laser-assisted selection of viable spermatozoa before intracytoplasmatic sperm injection in a couple with male primary cilia dyskinesia. Fertil. Steril. 2008, 89, e9–e12.
- Ozkavukcu, S.; Celik-Ozenci, C.; Konuk, E.; Atabekoglu, C. Live birth after Laser Assisted Viability Assessment (LAVA) to detect pentoxifylline resistant ejaculated immotile spermatozoa during ICSI in a couple with male Kartagener’s syndrome. Reprod. Biol. Endocrinol. 2018, 16, 10.
- Salman Yazdi, R.; Bakhshi, S.; Jannat Alipoor, F.; Akhoond, M.R.; Borhani, S.; Farrahi, F.; Lotfi Panah, M.; Sadighi Gilani, M.A. Effect of 830-nm diode laser irradiation on human sperm motility. Lasers Med. Sci. 2014, 29, 97–104.
- Montag, M.; Rink, K.; Delacrétaz, G.; van der Ven, H. Laser-induced immobilization and plasma membrane permeabilization in human spermatozoa. Hum. Reprod. 2000, 15, 846–852.
- Hoogendijk, C.F.; Kruger, T.F.; Bouic, P.J.; Henkel, R.R. A novel approach for the selection of human sperm using annexin V-binding and flow cytometry. Fertil. Steril. 2009, 91, 1285–1292.
- Grunewald, S.; Paasch, U.; Glander, H.J. Enrichment of non-apoptotic human spermatozoa after cryopreservation by immunomagnetic cell sorting. Cell Tissue Bank. 2001, 2, 127–133.
- Lee, T.H.; Liu, C.H.; Shih, Y.T.; Tsao, H.M.; Huang, C.C.; Chen, H.H.; Lee, M.S. Magnetic-activated cell sorting for sperm preparation reduces spermatozoa with apoptotic markers and improves the acrosome reaction in couples with unexplained infertility. Hum. Reprod. 2010, 25, 839–846.
- Degheidy, T.; Abdelfattah, H.; Seif, A.; Albuz, F.K.; Gazi, S.; Abbas, S. Magnetic activated cell sorting: An effective method for reduction of sperm DNA fragmentation in varicocele men prior to assisted reproductive techniques. Andrologia 2015, 47, 892–896.
- Nadalini, M.; Tarozzi, N.; Di Santo, M.; Borini, A. Annexin V magnetic-activated cell sorting versus swim-up for the selection of human sperm in ART: Is the new approach better than the traditional one? J. Assist. Reprod. Genet. 2014, 31, 1045–1051.
- Cayli, S.; Jakab, A.; Ovari, L.; Delpiano, E.; Celik-Ozenci, C.; Sakkas, D.; Ward, D.; Huszar, G. Biochemical markers of sperm function: Male fertility and sperm selection for ICSI. Reprod. Biomed. Online. 2003, 7, 462–468.
- Huszar, G.; Ozkavukcu, S.; Jakab, A.; Celik-Ozenci, C.; Sati, G.L.; Cayli, S. Hyaluronic acid binding ability of human sperm reflects cellular maturity and fertilizing potential: Selection of sperm for intracytoplasmic sperm injection. Curr. Opin. Obstet. Gynecol. 2006, 18, 260–267.
- Jakab, A.; Sakkas, D.; Delpiano, E.; Cayli, S.; Kovanci, E.; Ward, D.; Revelli, A.; Huszar, G. Intracytoplasmic sperm injection: A novel selection method for sperm with normal frequency of chromosomal aneuploidies. Fertil. Steril. 2005, 84, 1665–1673.
- Van Den Bergh, M.J.; Fahy-Deshe, M.; Hohl, M.K. Pronuclear zygote score following intracytoplasmic injection of hyaluronan-bound spermatozoa: A prospective randomized study. Reprod. Biomed. Online 2009, 19, 796–801.
- Engelmann, U.; Krassnigg, F.; Schatz, H.; Schill, W.B. Separation of human X and Y spermatozoa by free-flow electrophoresis. Gamete Res. 1988, 19, 151–160.
- Kheirollahi-Kouhestani, M.; Razavi, S.; Tavalaee, M.; Deemeh, M.R.; Mardani, M.; Moshtaghian, J.; Nasr-Esfahani, M.H. Selection of sperm based on combined density gradient and Zeta method may improve ICSI outcome. Hum. Reprod. 2009, 24, 2409–2416.
- Razavi, S.H.; Nasr-Esfahani, M.H.; Deemeh, M.R.; Shayesteh, M.; Tavalaee, M. Evaluation of zeta and HA-binding methods for selection of spermatozoa with normal morphology, protamine content and DNA integrity. Andrologia 2010, 42, 13–19.
- Nasr Esfahani, M.H.; Deemeh, M.R.; Tavalaee, M.; Sekhavati, M.H.; Gourabi, H. Zeta Sperm Selection Improves Pregnancy Rate and Alters Sex Ratio in Male Factor Infertility Patients: A Double-Blind, Randomized Clinical Trial. Int. J. Fertil. Steril. 2016, 10, 253–260.
- Chan, P.J.; Jacobson, J.D.; Corselli, J.U.; Patton, W.C. A simple zeta method for sperm selection based on membrane charge. Fertil. Steril. 2006, 85, 481–486.
- Ainsworth, C.; Nixon, B.; Aitken, R.J. Development of a novel electrophoretic system for the isolation of human spermatozoa. Hum. Reprod. 2005, 20, 2261–2270.
- Shalom-Paz, E.; Anabusi, S.; Michaeli, M.; Karchovsky-Shoshan, E.; Rothfarb, N.; Shavit, T.; Ellenbogen, A. Can intra cytoplasmatic morphologically selected sperm injection (IMSI) technique improve outcome in patients with repeated IVF-ICSI failure? a comparative study. Gynecol. Endocrinol. 2015, 31, 247–251.
- Leandri, R.D.; Gachet, A.; Pfeffer, J.; Celebi, C.; Rives, N.; Carre-Pigeon, F.; Kulski, O.; Mitchell, V.; Parinaud, J. Is intracytoplasmic morphologically selected sperm injection (IMSI) beneficial in the first ART cycle? a multicentric randomized controlled trial. Andrology 2013, 1, 692–697.
- Teixeira, D.M.; Hadyme Miyague, A.; Barbosa, M.A.; Navarro, P.A.; Raine-Fenning, N.; Nastri, C.O.; Martins, W.P. Regular (ICSI) versus ultra-high magnification (IMSI) sperm selection for assisted reproduction. Cochrane Database Syst. Rev. 2020, 2, CD010167.
- Fortunato, A.; Boni, R.; Leo, R.; Nacchia, G.; Liguori, F.; Casale, S.; Bonassisa, P.; Tosti, E. Vacuoles in sperm head are not associated with head morphology, DNA damage and reproductive success. Reprod. Biomed. Online 2016, 32, 154–161.
- Smith, G.D.; Takayama, S. Application of microfluidic technologies to human assisted reproduction. Mol. Hum. Reprod. 2017, 23, 257–268.
- Parrella, A.C.D.; Keating, D.; Rosenwaks, Z.; Palermo, G.D. A microfluidic devide for selecting the most progressively motile spermatozoa yields a higher rate of euploid embryos. Fertil. Steril. 2018, 110, e342.
- Baldini, D.; Baldini, A.; Silvestris, E.; Vizziello, G.; Ferri, D.; Vizziello, D. A fast and safe technique for sperm preparation in ICSI treatments within a randomized controlled trial (RCT). Reprod. Biol. Endocrinol. 2020, 18, 88.
- Palini, S.; Stefani, S.; Primiterra, M.; Benedetti, S.; Barone, S.; Carli, L.; Vaccari, E.; Murat, U.; Feichtinger, W. Comparison of in vitro fertilization outcomes in ICSI cycles after human sperm preparation by density gradient centrifugation and direct micro swim-up without centrifugation. JBRA Assist. Reprod. 2017, 21, 89–93.
- Ezeh, U.I.; Moore, H.D.; Cooke, I.D. A prospective study of multiple needle biopsies versus a single open biopsy for testicular sperm extraction in men with non-obstructive azoospermia. Hum. Reprod. 1998, 13, 3075–3080.
- Girardi, S.K.; Schlegel, P.N. Microsurgical epididymal sperm aspiration: Review of techniques, preoperative considerations, and results. J. Androl. 1996, 17, 5–9.
- Ozkocer, S.E.; Konac, E. The current perspective on genetic and epigenetic factors in sperm maturation in the epididymis. Andrologia 2021, 53, e13989.
- Rossato, M.; Di Virgilio, F.; Foresta, C. Involvement of osmo-sensitive calcium influx in human sperm activation. Mol. Hum. Reprod. 1996, 2, 903–909.
- Nowicka-Bauer, K.; Szymczak-Cendlak, M. Structure and Function of Ion Channels Regulating Sperm Motility—An Overview. Int. J. Mol. Sci. 2021, 22, 3259.
- Gianaroli, L.; Magli, M.C.; Collodel, G.; Moretti, E.; Ferraretti, A.P.; Baccetti, B. Sperm head’s birefringence: A new criterion for sperm selection. Fertil. Steril. 2008, 90, 104–112.
- Ghosh, S.; Chattopadhyay, R.; Bose, G.; Ganesh, A.; Das, S.; Chakravarty, B.N. Selection of birefringent spermatozoa under Polscope: Effect on intracytoplasmic sperm injection outcome. Andrologia 2012, 44 (Suppl. 1), 734–738.
- Morales, P.; Llanos, M.; Yovich, J.L.; Cummins, J.M.; Vigil, P. Pentoxifylline increases sperm penetration into zona-free hamster oocytes without increasing the acrosome reaction. Andrologia 1993, 25, 359–362.
- Mahaldashtian, M.; Khalili, M.A.; Nottola, S.A.; Woodward, B.; Macchiarelli, G.; Miglietta, S. Does in vitro application of pentoxifylline have beneficial effects in assisted male reproduction? Andrologia 2021, 53, e13722.
- Park, Y.J.; Pang, M.G. Mitochondrial Functionality in Male Fertility: From Spermatogenesis to Fertilization. Antioxidants 2021, 10, 98.
- Ebner, T.; Moser, M.; Tews, G. Possible applications of a non-contact 1.48 microm wavelength diode laser in assisted reproduction technologies. Hum. Reprod. Update 2005, 11, 425–435.
- Neri, Q.V.; Lee, B.; Rosenwaks, Z.; Machaca, K.; Palermo, G.D. Understanding fertilization through intracytoplasmic sperm injection (ICSI). Cell Calcium. 2014, 55, 24–37.
- Guse, A.H.; Gil Montoya, D.C.; Diercks, B.P. Mechanisms and functions of calcium microdomains produced by ORAI channels, d-myo-inositol 1,4,5-trisphosphate receptors, or ryanodine receptors. Pharmacol. Ther. 2021, 223, 107804.
- Berridge, M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016, 96, 1261–1296.
- Ceyhan, Y.; Zhang, M.; Guo, J.; Sandoval, C.G.; Vacher, J.; Kaftanovskaya, E.M.; Agoulnik, A.I.; Agoulnik, I.U. Deletion of inositol polyphosphate 4-phosphatase type-II B affects spermatogenesis in mice. PLoS ONE 2020, 15, e0233163.
- Nordhoff, V.; Schüring, A.N.; Krallmann, C.; Zitzmann, M.; Schlatt, S.; Kiesel, L.; Kliesch, S. Optimizing TESE-ICSI by laser-assisted selection of immotile spermatozoa and polarization microscopy for selection of oocytes. Andrology 2013, 1, 67–74.
- Shan, S.; Xu, F.; Hirschfeld, M.; Brenig, B. Sperm Lipid Markers of Male Fertility in Mammals. Int. J. Mol. Sci. 2021, 22, 8767.
- Hernández-Silva, G.; Fabián López-Araiza, J.E.; López-Torres, A.S.; Larrea, F.; Torres-Flores, V.; Chirinos, M. Proteomic characterization of human sperm plasma membrane-associated proteins and their role in capacitation. Andrology 2020, 8, 171–180.
- Plouffe, B.D.; Murthy, S.K.; Lewis, L.H. Fundamentals and application of magnetic particles in cell isolation and enrichment: A review. Rep. Prog. Phys. 2015, 78, 016601.
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutelingsperger, C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin, V. J. Immunol. Methods 1995, 184, 39–51.
- Arends, M.J.; Wyllie, A.H. Apoptosis: Mechanisms and roles in pathology. Int. Rev. Exp. Pathol. 1991, 32, 223–254.
- Dandekar, P.; Aggeler, J.; Talbot, P. Structure, distribution and composition of the extracellular matrix of human oocytes and cumulus masses. Hum. Reprod. 1992, 7, 391–398.
- Parmegiani, L.; Cognigni, G.E.; Ciampaglia, W.; Pocognoli, P.; Marchi, F.; Filicori, M. Efficiency of hyaluronic acid (HA) sperm selection. J. Assist. Reprod. Genet. 2010, 27, 13–16.
- Barak, Y.; Menezo, Y.; Veiga, A.; Elder, K. A physiological replacement for polyvinylpyrrolidone (PVP) in assisted reproductive technology. Hum. Fertil. 2001, 4, 99–103.
- Parmegiani, L.; Cognigni, G.E.; Bernardi, S.; Troilo, E.; Ciampaglia, W.; Filicori, M. “Physiologic ICSI”: Hyaluronic acid (HA) favors selection of spermatozoa without DNA fragmentation and with normal nucleus, resulting in improvement of embryo quality. Fertil. Steril. 2010, 93, 598–604.
- Yanagimachi, R.; Noda, Y.D.; Fujimoto, M.; Nicolson, G.L. The distribution of negative surface charges on mammalian spermatozoa. Am. J. Anat. 1972, 135, 497–519.
- Zarei-Kheirabadi, M.; Shayegan Nia, E.; Tavalaee, M.; Deemeh, M.R.; Arabi, M.; Forouzanfar, M.; Javadi, G.R.; Nasr-Esfahani, M.H. Evaluation of ubiquitin and annexin V in sperm population selected based on density gradient centrifugation and zeta potential (DGC-Zeta). J. Assist. Reprod. Genet. 2012, 29, 365–371.
- Zahedi, A.; Tavalaee, M.; Deemeh, M.R.; Azadi, L.; Fazilati, M.; Nasr-Esfahani, M.H. Zeta potential vs apoptotic marker: Which is more suitable for ICSI sperm selection? J. Assist. Reprod. Genet. 2013, 30, 1181–1186.
- Bartoov, B.; Berkovitz, A.; Eltes, F. Selection of spermatozoa with normal nuclei to improve the pregnancy rate with intracytoplasmic sperm injection. N. Engl. J. Med. 2001, 345, 1067–1068.
- Lo Monte, G.; Murisier, F.; Piva, I.; Germond, M.; Marci, R. Focus on intracytoplasmic morphologically selected sperm injection (IMSI): A mini-review. Asian J. Androl. 2013, 15, 608–615.
- Franco, J.G., Jr.; Baruffi, R.L.; Mauri, A.L.; Petersen, C.G.; Oliveira, J.B.; Vagnini, L. Significance of large nuclear vacuoles in human spermatozoa: Implications for ICSI. Reprod. Biomed. Online 2008, 17, 42–45.
- Miki, K.; Clapham, D.E. Rheotaxis guides mammalian sperm. Curr. Biol. 2013, 23, 443–452.
- Hino, T.; Yanagimachi, R. Active peristaltic movements and fluid production of the mouse oviduct: Their roles in fluid and sperm transport and fertilization. Biol. Reprod. 2019, 101, 40–49.
- Nagata, M.P.B.; Endo, K.; Ogata, K.; Yamanaka, K.; Egashira, J.; Katafuchi, N.; Yamanouchi, T.; Matsuda, H.; Goto, Y.; Sakatani, M.; et al. Live births from artificial insemination of microfluidic-sorted bovine spermatozoa characterized by trajectories correlated with fertility. Proc. Natl. Acad. Sci. USA 2018, 115, E3087–E3096.
- De Martin, H.; Cocuzza, M.S.; Tiseo, B.C.; Wood, G.J.A.; Miranda, E.P.; Monteleone, P.A.A.; Soares, J.M., Jr.; Serafini, P.C.; Srougi, M.; Baracat, E.C. Positive rheotaxis extended drop: A one-step procedure to select and recover sperm with mature chromatin for intracytoplasmic sperm injection. J. Assist. Reprod. Genet. 2017, 34, 1699–1708.
- Oren-Benaroya, R.; Orvieto, R.; Gakamsky, A.; Pinchasov, M.; Eisenbach, M. The sperm chemoattractant secreted from human cumulus cells is progesterone. Hum. Reprod. 2008, 23, 2339–2345.
- Gatica, L.V.; Guidobaldi, H.A.; Montesinos, M.M.; Teves, M.E.; Moreno, A.I.; Uñates, D.R.; Molina, R.I.; Giojalas, L.C. Picomolar gradients of progesterone select functional human sperm even in subfertile samples. Mol. Hum. Reprod. 2013, 19, 559–569.
- Li, K.; Li, R.; Ni, Y.; Sun, P.; Liu, Y.; Zhang, D.; Huang, H. Novel distance-progesterone-combined selection approach improves human sperm quality. J. Transl. Med. 2018, 16, 203.
- Bahat, A.; Eisenbach, M. Sperm thermotaxis. Mol. Cell Endocrinol. 2006, 252, 115–119.
- Bahat, A.; Tur-Kaspa, I.; Gakamsky, A.; Giojalas, L.C.; Breitbart, H.; Eisenbach, M. Thermotaxis of mammalian sperm cells: A potential navigation mechanism in the female genital tract. Nat Med. 2003, 9, 149–150.
- Bahat, A.; Caplan, S.R.; Eisenbach, M. Thermotaxis of human sperm cells in extraordinarily shallow temperature gradients over a wide range. PLoS ONE 2012, 7, e41915.
- Pérez-Cerezales, S.; Laguna-Barraza, R.; de Castro, A.C.; Sánchez-Calabuig, M.J.; Cano-Oliva, E.; de Castro-Pita, F.J.; Montoro-Buils, L.; Pericuesta, E.; Fernández-González, R.; Gutiérrez-Adán, A. Sperm selection by thermotaxis improves ICSI outcome in mice. Sci. Rep. 2018, 8, 2902.
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189.
- Shirota, K.; Yotsumoto, F.; Itoh, H.; Obama, H.; Hidaka, N.; Nakajima, K.; Miyamoto, S. Separation efficiency of a microfluidic sperm sorter to minimize sperm DNA damage. Fertil. Steril. 2016, 105, 315–321.e1.
- Ma, R.; Xie, L.; Han, C.; Su, K.; Qiu, T.; Wang, L.; Huang, G.; Xing, W.; Qiao, J.; Wang, J.; et al. In vitro fertilization on a single-oocyte positioning system integrated with motile sperm selection and early embryo development. Anal. Chem. 2011, 83, 2964–2970.
- Simon, L.; Murphy, K.; Aston, K.I.; Emery, B.R.; Hotaling, J.M.; Carrell, D.T. Optimization of microelectrophoresis to select highly negatively charged sperm. J. Assist. Reprod. Genet. 2016, 33, 679–688.
- Eravuchira, P.J.; Mirsky, S.K.; Barnea, I.; Levi, M.; Balberg, M.; Shaked, N.T. Individual sperm selection by microfluidics integrated with interferometric phase microscopy. Methods 2018, 136, 152–159.
- Amaral, S.; Da Costa, R.; Wübbeling, F.; Redmann, K.; Schlatt, S. Raman micro-spectroscopy analysis of different sperm regions: A species comparison. Mol. Hum. Reprod. 2018, 24, 185–202.
- Di Caprio, G.; Ferrara, M.A.; Miccio, L.; Merola, F.; Memmolo, P.; Ferraro, P.; Coppola, G. Holographic imaging of unlabelled sperm cells for semen analysis: A review. J. Biophotonics 2015, 8, 779–789.
- de Wagenaar, B.; Geijs, D.J.; de Boer, H.; Bomer, J.G.; Olthuis, W.; van den Berg, A.; Segerink, L.I. Spermometer: Electrical characterization of single boar sperm motility. Fertil. Steril. 2016, 106, 773–780.e6.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
10 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




