
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohammad Main Uddin | + 2986 word(s) | 2986 | 2021-12-31 04:37:13 | | | |
| 2 | Lindsay Dong | Meta information modification | 2986 | 2022-01-10 03:36:04 | | | | |
| 3 | Lindsay Dong | Meta information modification | 2986 | 2022-01-10 03:36:32 | | |
Video Upload Options
Aquatic ecosystems are contaminated with heavy metals by natural and anthropogenic sources. Among heavy metals, cadmium (Cd), arsenic (As), chromium (Cr), lead (Pb), and mercury (Hg) cause significant damage to aquatic ecosystems and can invariably affect human health. These metals can enter the human body through food chains, and the presence of heavy metals in food can lead to numerous human health consequences. Heavy metals in aquatic plants can affect plant physicochemical functions, growth, and crop yield.
1. Introduction
2. Heavy Metals Present/Accumulated in Rice and Aquatic Plants
Aquatic macrophytes play a pivotal role in the nutrient recycling and aerobic or anaerobic conditions of the water bodies they are present in [7]. They have the remarkable capability of absorbing nutrients and pollutants, accumulating them in their tissues, and growing in unfavourable conditions [8]. Heavy metals are one of the most serious offenders of polluting aquatic systems, mainly due to their toxicity, persistence in the environment, and incorporation into food chains [9].
Some aquatic food sources have shown to be reliable sources of treating contaminated land. For example, Eleocharis dulcis (Chinese water chestnut) is used to treat uranium mine runoff in Australia [10], and Neptunia oleracea (water mimosa) has been identified as a feasible phytoremediator to clean aquatic systems contaminated with arsenic (As) [11]. Despite the advantages of being able to use aquatic plants to remove heavy metals, the disadvantage stands in the high probability of it being harmful to humans by entering food chains or direct consumption.
As the most abundantly prevalent aquatic food source, the accumulation of heavy metals on rice plants can have widespread repercussions. Due to their ability to adapt to waterlogged or submerged conditions by forming special air channels called aerenchyma which allow O2 transport to submerged tissues, contamination of irrigation water can lead to accumulation of heavy metal in rice plants. Sharma et al. [12] summarises some recent studies from various locations worldwide on the contaminations of rice grains with potentially toxic elements and exemplifies a range of elements including lead (Pb), cadmium (Cd), chromium (Cr), iron (Fe), zinc (Zn), arsenic (As), uranium (U), thorium (Th), copper (Cu), nickel (Ni), molybdenum (Mo), manganese (Mn), barium (Ba), and antimony (Sb).
In most nations, rapid economic development and urbanisation can lead to attempts by people to combine traditional cultivation methods with urbanised practices, often using unsuitable urban environments for cultivating crops. Cultivation of aquatic plants for human consumption in water bodies that are contaminated with pollutants and heavy metals can lead to accumulation of metals to various degrees within the plants.
Some aquatic plants tend to bioaccumulate metals depending on their initial concentration in waters. In such instances, the types of metals accumulated may vary depending on the plant species, as seen in Nasturtium officinale (watercress) which tend to accumulate Cd, Cr, and Co in different concentrations [13].
3. Mechanisms of Heavy Metal Accumulation
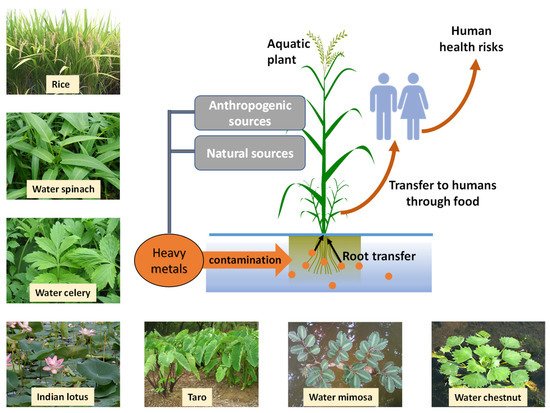
4. Sources of Heavy Metals for Bioaccumulation
5. Heavy Metals in Food Chain from Rice and Aquatic Plants to Humans
-
Indicator plants—plants which are usually sensitive to heavy metals. These can be used as indicators as for the presence of metal in the substrate they have grown in.
-
Excluders—these plants can tolerate heavy metals in the substrate up to a threshold concentration. This is achieved by preventing the accumulation of the heavy metal in the cell by either blocking the uptake in roots or by energy dependent efflux pumps. Most metal (hyper) tolerant plants are categorised into this group.
-
Hyperaccumulators—in addition to the ability to tolerate high concentrations of specific elements, these plants can actively take them up and accumulate them in their aerial parts. Often these plants have specific mechanisms to avoid poisoning themselves by the accumulated metals.
| Element | Main Oxidising States | Natural and Lithogenic Sources | Anthropogenic Sources | Effects on Humans |
|---|---|---|---|---|
| Arsenic | As(III), As(V) | Weathering of rocks, volcanic eruptions, microbial colonization, As bearing minerals in the lithosphere (e.g., FeAsS, CoAsS, NiAs, AsS, As2S, As2O3) | Fossil fuel combustion, mining, smelting, fertilisers, glass production, chemotherapeutic drug production | Carcinogenic and neurotoxic |
| Cadmium | Cd(II) | Volcanic activities, weathering, erosion, wildfire, sea salt spray, dust storm, Cd bearing compounds in the lithosphere (e.g., CdS, CdCO3, Cu4Cd(SO4)2(OH)6.4H2O, CdSe) | Ni–Cd batteries, fossil fuel combustions, mining, cement production, plastic stabilisers, coatings industry, phosphate fertiliser | Carcinogenic |
| Chromium | Cr(III), Cr(VI) | Tectonic and hydrothermal events, in the lithosphere as FeCr2O4 and PbCrO4 | Aircraft industry, electroplating, wood preservation, tanning, mining, textile dyes manufacturing, metal corrosion inhibition, and cleaning of glassware | Carcinogenic and Mutagenic |
| Lead | Pb(II), Pb(IV) | Natural fires, natural deposits, sea salt spray, and volcanic eruptions and over 100 Pb-containing minerals in the lithosphere (e.g., PbS, PbCrO4, PbSO4, Pb5(PO4)3Cl, PbMn8O16, PbCO3) | Pb–acid battery recycling (PABC), Pb-containing gasoline in petrol, pipes, pesticides, ammunition, electronic wastes, mining, ore processing, pigment in paints, dyes, and ceramic glazes | Neurotoxic |
| Mercury | Hg, Hg(I), Hg(II) | Weathering of rock, volcanic eruptions, degassing and wildfire. In the lithosphere as metallic form (Hg)(0) (rare) or as HgS, Hg3S2Cl2, HgSb4S8 | Coal combustion, production of non-ferrous and ferrous metals, artisanal and small-scale gold mining (ASGM), cement production, pesticides, and fertilisers production | Neurotoxic |
6. Human Health Risk Associated with Heavy Metal Accumulation in Food
| Heavy Metal | Target Organ | Disease Condition/Clinical Effect | References |
|---|---|---|---|
| Arsenic | Nervous system, skin, pulmonary, gastrointestinal | Nausea, vomiting, multi-organ dysfunction syndrome, long QT syndrome, ‘rice water’ diarrhoea, nasal septum perforation, peripheral neuropathy, encephalopathy, respiratory cancer, skin cancer, prostate cancer, hypopigmentation, | [44][45] |
| Cadmium | Skeletal, renal, pulmonary | Osteomalacia, proteinuria, glucosuria, emphysema, pneumonitis, inhibition of progesterone and oestradiol, alterations in uterus, ovaries and oviduct, progesterone synthesis of ovaries, endocrine disruption, acting as estrogen in breast cancer, excess risk of cardiovascular mortality | [46][47] |
| Chromium | Pulmonary, gastrointestinal | Nasal septum perforation, respiratory cancer, ulcers, gastrointestinal haemorrhage, haemolysis, acute renal failure, pulmonary fibrosis, DNA damage | [48][49] |
| Lead | Nervous system, renal, hematopoietic system, gastrointestinal | Encephalopathy, anaemia, central nervous disorders, peripheral neuropathy, nausea, vomiting, abdominal pain, nephropathy, foot-drop/wrist-drop, damages circulatory system and cardiovascular system | [50][51] |
| Mercury | Nervous system, renal, gastrointestinal | Proteinuria, fever, vomiting, diarrhea, acute lung injury, nausea, metallic taste, gingivo-stomatitis, tremor, neurasthenia, nephrotic syndrome; hypersensitivity, cough, fever, tremor, malaise, motor neuropathy, gum disease, delusions and hallucinations | [52][53] |
7. Mitigation of Heavy Metal Accumulation in Rice and Aquatic Plants

References
- Hawkes, S.J. What is a “heavy metal”? J. Chem. Educ. 1997, 74, 1374.
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216.
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42.
- Aasim, M.; Bakhsh, A.; Sameeullah, M.; Karataş, M.; Khawar, K.M. Aquatic plants as human food. In Global Perspectives on Underutilized Crops; Ozturk, M., Hakeem, K., Ashraf, M., Ahmad, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 165–187.
- Mishra, P.; Mishra, M. Risk Assessment of heavy metal contamination in paddy soil, plants, and grains (Oryza sativa L.). In Environmental Pollution of Paddy Soils; Hashmi, M., Varma, A., Eds.; Springer: Cham, Switzerland, 2018; Volume 53, pp. 165–178.
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy metal contamination: An alarming threat to environment and human health. In Environmental Biotechnology: For Sustainable Future; Sobti, R., Arora, N., Kothari, R., Eds.; Springer: Singapore, 2019; pp. 103–125.
- Barko, J.W.; James, W.F. Effects of submerged aquatic macrophytes on nutrient dynamics, sedimentation, and resuspension. In The Structuring Role of Submerged Macrophytes in Lakes; Ecological Studies (Analysis and Synthesis); Jeppesen, E., Søndergaard, M., Søndergaard, M., Christoffersen, K., Eds.; Springer: New York, NY, USA, 1998; Volume 131, pp. 197–214.
- Ali, S.; Abbas, Z.; Rizwan, M.; Zaheer, I.E.; Yavaş, İ.; Ünay, A.; Abdel-Daim, M.M.; Bin-Jumah, M.; Hasanuzzaman, M.; Kalderis, D. Application of floating aquatic plants in phytoremediation of heavy metals polluted water: A review. Sustainability 2020, 12, 1927.
- Proshad, R.; Kormoker, T.; Mursheed, N.; Islam, M.M.; Bhuyan, M.I.; Islam, M.S.; Mithu, T.N. Heavy metal toxicity in agricultural soil due to rapid industrialization in Bangladesh: A review. Int. J. Adv. Geosci. 2018, 6, 83–88.
- Overall, R.A.; Parry, D.L. The uptake of uranium by Eleocharis dulcis (Chinese water chestnut) in the Ranger Uranium Mine constructed wetland filter. Environ. Pollut. 2004, 132, 307–320.
- Atabaki, N.; Shaharuddin, N.A.; Ahmad, S.A.; Nulit, R.; Abiri, R. Assessment of Water Mimosa (Neptunia oleracea Lour.) Morphological, Physiological, and Removal Efficiency for Phytoremediation of Arsenic-Polluted Water. Plants 2020, 9, 1500.
- Sharma, S.; Kaur, I.; Nagpal, A.K. Contamination of rice crop with potentially toxic elements and associated human health risks—A review. Environ. Sci. Pollut. Res. 2021, 28, 1–18.
- Duman, F.; Leblebici, Z.; Aksoy, A. Growth and bioaccumulation characteristics of watercress (Nasturtium officinale R. BR.) exposed to cadmium, cobalt and chromium. Chem. Speciat. Bioavailab. 2009, 21, 257–265.
- Lee, S.; Kang, D.-W.; Yoo, J.-H.; Park, S.-W.; Oh, K.-S.; Lee, J.-H.; Cho, I.K.; Moon, B.-C.; Kim, W.-I. Determination of bioconcentration factor of heavy metal (loid)s in rice grown on soils vulnerable to heavy metal (loid)s contamination. Korean J. Soil. Sci. Fert. 2017, 50, 106–114.
- Raskin, I.; Smith, R.D.; Salt, D.E. Phytoremediation of metals: Using plants to remove pollutants from the environment. Curr. Opin. Biotechnol. 1997, 8, 221–226.
- Nyquist, J.; Greger, M. Uptake of Zn, Cu, and Cd in metal loaded Elodea canadensis. Environ. Exp. Bot. 2007, 60, 219–226.
- Greger, M. Metal availability and bioconcentration in plants. In Heavy Metal Stress in Plants; Springer: Berlin/Heidelberg, Gremany, 1999; pp. 1–27.
- Gallego, S.M.; Benavides, M.P.; Tomaro, M.L. Effect of heavy metal ion excess on sunflower leaves: Evidence for involvement of oxidative stress. Plant Sci. 1996, 121, 151–159.
- Salt, D.E.; Blaylock, M.; Kumar, N.P.; Dushenkov, V.; Ensley, B.D.; Chet, I.; Raskin, I. Phytoremediation: A novel strategy for the removal of toxic metals from the environment using plants. Bio/Technology 1995, 13, 468–474.
- Neeratanaphan, L.; Khamma, S.; Benchawattananon, R.; Ruchuwararak, P.; Appamaraka, S.; Intamat, S. Heavy metal accumulation in rice (Oryza sativa) near electronic waste dumps and related human health risk assessment. Hum. Ecol. Risk Assess. Int. J. 2017, 23, 1086–1098.
- Fan, Y.; Li, Y.; Li, H.; Cheng, F. Evaluating heavy metal accumulation and potential risks in soil-plant systems applied with magnesium slag-based fertilizer. Chemosphere 2018, 197, 382–388.
- Edelstein, M.; Ben-Hur, M. Heavy metals and metalloids: Sources, risks and strategies to reduce their accumulation in horticultural crops. Sci. Hortic. 2018, 234, 431–444.
- Leitenmaier, B.; Küpper, H. Compartmentation and complexation of metals in hyperaccumulator plants. Front. Plant Sci. 2013, 4, 374.
- Nazir, R.; Khan, M.; Masab, M.; Rehman, H.U.; Rauf, N.U.; Shahab, S.; Ameer, N.; Sajed, M.; Ullah, M.; Rafeeq, M. Accumulation of heavy metals (Ni, Cu, Cd, Cr, Pb, Zn, Fe) in the soil, water and plants and analysis of physico-chemical parameters of soil and water collected from Tanda Dam Kohat. J. Pharm. Sci. Res. 2015, 7, 89.
- Gladyshev, M.; Gribovskaya, I.; Ivanova, E.; Moskvichova, A.; Muchkina, E.Y.; Chuprov, S. Metal concentrations in the ecosystem and around recreational and fish-breeding pond Bugach. Water Resour. 2001, 28, 288–296.
- Hapke, H.-J. Heavy metal transfer in the food chain to humans. In Fertilizers and Environment. Developments in Plant and Soil Sciences; Springer: Dordrecht, The Netherlands, 1996; Volume 66, pp. 431–436.
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 1–21.
- Yu, Z.; Gunn, L.; Wall, P.; Fanning, S. Antimicrobial resistance and its association with tolerance to heavy metals in agriculture production. Food Microbiol. 2017, 64, 23–32.
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176.
- Huang, Y.; Wang, L.; Wang, W.; Li, T.; He, Z.; Yang, X. Current status of agricultural soil pollution by heavy metals in China: A meta-analysis. Sci. Total Environ. 2019, 651, 3034–3042.
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and health effects of heavy metal toxicity in humans. In Poisoning in the Modern World-New Tricks for an Old Dog; BoD–Books on Demand: Norderstedt, Germany, 2019; Volume 10.
- Ugulu, I.; Ahmad, K.; Khan, Z.I.; Munir, M.; Wajid, K.; Bashir, H. Effects of organic and chemical fertilizers on the growth, heavy metal/metalloid accumulation, and human health risk of wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 2021, 28, 12533–12545.
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197.
- Izah, S.C.; Chakrabarty, N.; Srivastav, A.L. A review on heavy metal concentration in potable water sources in Nigeria: Human health effects and mitigating measures. Expos. Health 2016, 8, 285–304.
- Vigneri, R.; Malandrino, P.; Gianì, F.; Russo, M.; Vigneri, P. Heavy metals in the volcanic environment and thyroid cancer. Mol. Cell. Endocrinol. 2017, 457, 73–80.
- Sabath, E.; Robles-Osorio, M.L. Renal health and the environment: Heavy metal nephrotoxicity. Nefrología 2012, 32, 279–286.
- Otitoju, O.; Otitoju, G.; Iyeghe, L.; Onwurah, I. Quantification of heavy metals in some locally produced rice (Oryza sativa) from the northern region of Nigeria. J. Environ. Earth Sci. 2014, 4, 67–71.
- Dapul, H.; Laraque, D. Lead poisoning in children. Adv. Pediatr. 2014, 61, 313–333.
- Peralta-Videa, J.R.; Lopez, M.L.; Narayan, M.; Saupe, G.; Gardea-Torresdey, J. The biochemistry of environmental heavy metal uptake by plants: Implications for the food chain. Int. J. Biochem. Cell Biol. 2009, 41, 1665–1677.
- Rahman, M.M.; Owens, G.; Naidu, R. Arsenic levels in rice grain and assessment of daily dietary intake of arsenic from rice in arsenic-contaminated regions of Bangladesh—Implications to groundwater irrigation. Environ. Geochem. Health 2009, 31, 179–187.
- Oberoi, S.; Barchowsky, A.; Wu, F. The global burden of disease for skin, lung, and bladder cancer caused by arsenic in food. Cancer Epidemiol. Prev. Biomark. 2014, 23, 1187–1194.
- Mandal, P. An insight of environmental contamination of arsenic on animal health. Emerg. Contam. 2017, 3, 17–22.
- Karagas, M.R.; Punshon, T.; Davis, M.; Bulka, C.M.; Slaughter, F.; Karalis, D.; Argos, M.; Ahsan, H. Rice intake and emerging concerns on arsenic in rice: A review of the human evidence and methodologic challenges. Curr. Environ. Health Rep. 2019, 6, 361–372.
- Ötleş, S.; Çağındı, Ö. Health importance of arsenic in drinking water and food. Environ. Geochem. Health 2010, 32, 367–371.
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107.
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Moghadamnia, A.A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135.
- Himeno, S.; Aoshima, K. Cadmium Toxicity: New Aspects in Human Disease, Rice Contamination, and Cytotoxicity; Springer: Singapore, 2019.
- Dattilo, A.M.; Miguel, S.G. Chromium in health and disease. Nut. Today 2003, 38, 121–133.
- Onakpa, M.M.; Njan, A.A.; Kalu, O.C. A review of heavy metal contamination of food crops in Nigeria. Ann. Glob. Health 2018, 84, 488.
- Papanikolaou, N.C.; Hatzidaki, E.G.; Belivanis, S.; Tzanakakis, G.N.; Tsatsakis, A.M. Lead toxicity update. A brief review. Med. Sci. Monit. 2005, 11, RA329.
- Wani, A.; Ara, A.; Usmani, J. Lead toxicity: A review. Interdiscipl. Toxicol. 2015, 8, 55–64.
- Zahir, F.; Rizwi, S.J.; Haq, S.K.; Khan, R.H. Low dose mercury toxicity and human health. Environ. Toxicol. Pharmacol. 2005, 20, 351–360.
- Mousavi, A.; Chávez, R.D.; Ali, A.-M.S.; Cabaniss, S.E. Mercury in natural waters: A mini-review. Environ. Forensics 2011, 12, 14–18.
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Not. 2011, 2011, 402647.
- Bhagwat, V.R. Safety of Water Used in Food Production. In Food Safety and Human Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 219–247.
- Stoop, W.A.; Uphoff, N.; Kassam, A. A review of agricultural research issues raised by the system of rice intensification (SRI) from Madagascar: Opportunities for improving farming systems for resource-poor farmers. Agric. Syst. 2002, 71, 249–274.
- Lawler, S.P. Rice fields as temporary wetlands: A review. Isr. J. Zool. 2001, 47, 513–528.
- Sundaram, L.; Rajendran, S.; Subramanian, N. Metal stress impacting plant growth in contaminated soil is alleviated by microbial siderophores. In Role of Microbial Communities for Sustainability. Microorganisms for Sustainability; Seneviratne, G., Zavahir, J.S., Eds.; Springer: Singapore, 2021; Volume 29, pp. 317–332.
- Roba, C.; Roşu, C.; Piştea, I.; Ozunu, A.; Baciu, C. Heavy metal content in vegetables and fruits cultivated in Baia Mare mining area (Romania) and health risk assessment. Environ. Sci. Pollut. Res. 2016, 23, 6062–6073.
- Chaturvedi, A.D.; Pal, D.; Penta, S.; Kumar, A. Ecotoxic heavy metals transformation by bacteria and fungi in aquatic ecosystem. World J. Microbiol. Biotechnol. 2015, 31, 1595–1603.
- Hejna, M.; Gottardo, D.; Baldi, A.; Dell’Orto, V.; Cheli, F.; Zaninelli, M.; Rossi, L. Nutritional ecology of heavy metals. Animal 2018, 12, 2156–2170.
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of soil pollution by heavy metals and their accumulation in vegetables: A review. Water Air Soil Pollut. 2019, 230, 1–9.
- Sumiahadi, A.; Acar, R. A review of phytoremediation technology: Heavy metals uptake by plants. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 4th International Conference on Sustainable Agriculture and Environment (4th ICSAE) Surakarta, Indonesia, 10–12 August 2017; IOP Publishing: Bristol, UK, 2018; Volume 142, p. 012023.





