
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jurg Arthur Schutz | + 1419 word(s) | 1419 | 2022-01-07 07:09:53 | | | |
| 2 | Yvaine Wei | Meta information modification | 1419 | 2022-01-10 03:15:00 | | |
Video Upload Options
Disposable respirator masks with an accepted performance rating are seriously compromised from an exposure to saturated alcoholic vapours, can tolerate a one-off spray treatment with an alcoholic solution and retain their attested protection under the influence of alcoholic vapours from the use of hand sanitizer or spray sanitizer.
1. Introduction
The most common sanitizing treatments include alcoholic gel sanitizer for hand care and the spraying of alcoholic solutions onto table surfaces, door handles, keyboards, etc. to suppress the spread of viral loads via fomites, particularly where low ambient temperatures significantly slow the natural decay of coronaviruses [1][2]. Common products have a minimum of 60% alcohol content following recommendations by the Centre for Disease Control CDC [3] or even higher according to the World Health Organization WHO [4].
Another important tool in combatting the spread of disease is the use of face masks [5][6][7][8]. The COVID-19 pandemic has led, predictably, to significant face mask supply shortages due to high demand world-wide. Finding replacements for face mask materials is important and particularly difficult because common alternatives do not provide the necessary high-level protection and acceptable breathability at the same time [9].
Another way to overcome supply shortages, which is often practiced in areas of significant disease outbreaks, is the reuse of disposable face mask after subjecting them to specialized treatments that, in essence, maintain the performance of the PPE [10][11][12][13] while deactivating the virus captured on the masks [14].
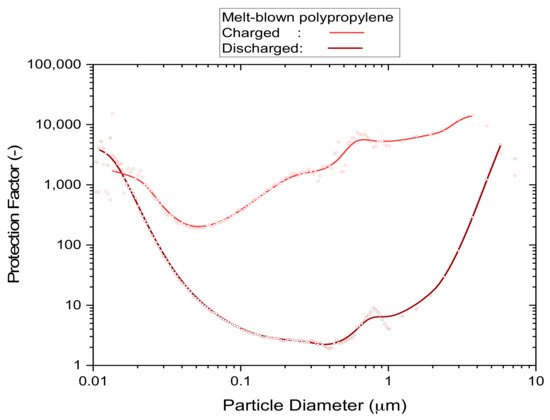
Filter media that are either pleated or contain high levels of electrostatic charge achieve high protection levels from particles of all sizes. The reason for this is that at least one of these enhancement techniques is required to achieve an acceptable level of filtration performance under the typical constraints for face masks of size, weight and flow resistance. Disposable respirators, for instance, all work with electrostatic attraction to capture particles in a size range of approximately 0.05–2 µm, as shown in the example of Figure 1, which relates the particle concentration outside of the mask to the concentration that is breathed in (according to the definition of ‘protection factor’). These are the type of particles that are most difficult to capture because the normally predominant processes, which are based on fluid dynamics, are ineffective in this size range [15][16][17] (brown curve in Figure 1). It is the electrostatic field contained in these filter materials that captures aerosol particles almost exclusively in this sub-micrometre size range, rather than the physically visible filter material that acts as a screen. This is relevant because the actual size of coronaviruses is in the sub-micrometre size range, and simple activities such as breathing and talking alone already bear the potential to generate sub-micrometre aerosols from viral loads [18].
2. Exposure to Saturated Alcohol Vapor
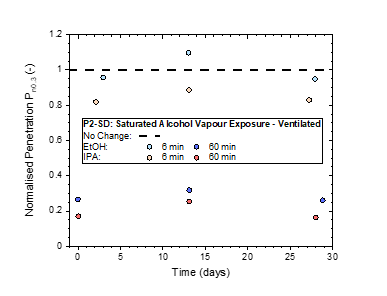

3. Exposure to Vapours from Hand Sanitization or Cleaning of Exposed Surfaces
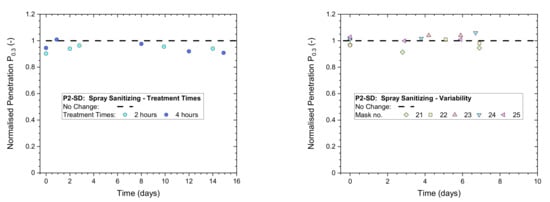
4. Exposure to Mask Sanitization
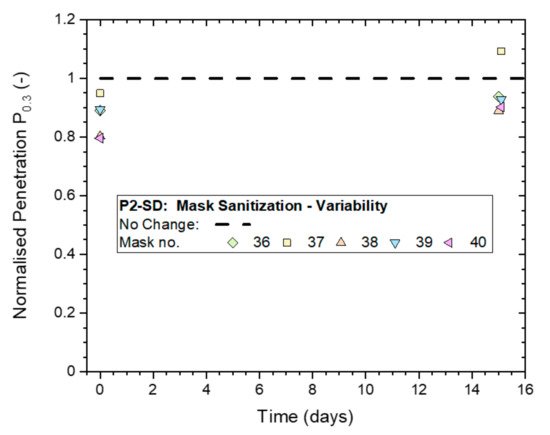
Results support the conclusion that the filtration performance of some masks, at least, may have deteriorated as a result of the mask sanitization treatment.
5. Conclusions
The effects of alcoholic vapours (ethanol and isopropanol) released during use of hand sanitizers or alcoholic sprays on the protective properties of common disposable respirator masks has been investigated.
The most severe challenge involved exposing the masks to saturated alcoholic vapours, similar to the conditioning methods [33] typically used for the certification of air-conditioning products. Results revealed a large deterioration in particulate protection for exposures of 60 min or longer, with the deterioration easing to insignificant as the exposure period was reduced to 6 min.
The other three challenge types were designed to represent more real-world exposure scenarios, which people might experience when wearing respirator masks indoors in hospitals, office spaces, shopping centres and the like. The chosen experimental settings included ‘worst-case’ scenarios based on an ‘experience’ level that may be encountered during attempts to mitigate exposure to the SARS-CoV-2 virus:
- The use of gel hand sanitizer, as commonly provided in many places by automatic or manual dispenser units, to maintain good personal hygiene;
- The spraying of 70% isopropanol solution on table surfaces for general hygiene purposes and for the removal of fomites;
- Sanitizing respirators by spraying small amounts of 70% isopropanol solution directly onto the face of the mask.
Results from heavy exposures showed respirator masks retained excellent protection performance in all three of these scenarios, while caution must be exercised with multiple applications of mask sanitization (scenario 3) as this may deteriorate mask performance and the reuse of disposable masks is not recommended by manufacturers.
References
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020, 17, 145.
- Biryukov, J.; Boydston, J.A.; Dunning, R.A.; Yeager, J.J.; Wood, S.; Reese, A.L.; Ferris, A.; Miller, D.; Weaver, W.; Zeitouni, N.E.; et al. Increasing Temperature and Relative Humidity Accelerates Inactivation of SARS-CoV-2 on Surfaces. mSphere 2020, 5, e00441-20.
- National Center for Immunization and Respiratory Diseases—Division of Viral Diseases (NCIRD). Hand Hygiene Recommendations, Guidance for Healthcare Providers about Hand Hygiene and COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/hand-hygiene.html (accessed on 17 May 2020).
- WHO. Guide to Local Production: WHO-Recommended Handrub Formulations; WHO: Geneva, Switzerland, 2010; p. 9. Available online: https://www.who.int/gpsc/5may/Guide_to_Local_Production.pdf (accessed on 11 February 2021).
- Freitag, S.; Howell, S.G.; Jim, K.T.C. Why simple face masks are unexpectedly efficient in reducing viral aerosol transmissions. medRxiv 2020, 19.
- Asadi, S.; Bouvier, N.; Wexler, A.S.; Ristenpart, W.D. The coronavirus pandemic and aerosols: Does COVID-19 transmit via expiratory particles? Aerosol Sci. Technol. 2020, 54, 635–638.
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567.
- Booth, T.F.; Kournikakis, B.; Bastien, N.; Ho, J.; Kobasa, D.; Stadnyk, L.; Li, Y.; Spence, M.; Paton, S.; Henry, B.; et al. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J. Infect. Dis. 2005, 191, 1472–1477.
- Sousan, S.; Garcia, N.; White, A.; Balanay, J.A. Filtration efficiency of surgical sterilization fabric for respiratory protection during COVID-19 pandemic. Am. J. Infect. Control 2021, 49, 1–7.
- Rubio-Romero, J.C.; Pardo-Ferreira, M.d.C.; Torrecilla-García, J.A.; Calero-Castro, S. Disposable masks: Disinfection and sterilization for reuse, and non-certified manufacturing, in the face of shortages during the COVID-19 pandemic. Saf. Sci. 2020, 129, 104830.
- Liao, L.; Xiao, W.; Zhao, M.; Yu, X.; Wang, H.; Wang, Q.; Chu, S.; Cui, Y. Can N95 Respirators Be Reused after Disinfection? How Many Times? ACS Nano 2020, 14, 6348–6356.
- Grinshpun, S.A.; Yermakov, M.; Khodoun, M. Autoclave sterilization and ethanol treatment of re-used surgical masks and N95 respirators during COVID-19: Impact on their performance and integrity. J. Hosp. Infect. 2020, 105, 608–614.
- Lin, T.-H.; Tseng, C.-C.; Huang, Y.-L.; Lin, H.-C.; Lai, C.-Y.; Lee, S.-A. Effectiveness of N95 Facepiece Respirators in Filtering Aerosol Following Storage and Sterilization. Aerosol Air Qual. Res. 2020, 20, 833–843.
- Fischer, R.; Morris, D.; van Doremalen, N.; Sarchette, S.; Matson, M.J.; Bushmaker, T.; Yinda, C.K.; Seifert, S.; Gamble, A.; Williamson, B.; et al. Effectiveness of N95 Respirator Decontamination and Reuse against SARS-CoV-2 Virus. Emerg. Infect. Dis. J. 2020, 26, 2253.
- Baumgartner, H.P.; Löffler, F. The Collection Performance of Electret Filters in the Particle-Size Range 10 Nm-10 Mu-M. J. Aerosol Sci. 1986, 17, 438–445.
- Lethimäki, M.; Heinonen, K. Reliability of Electret Filters. Build. Environ. 1994, 29, 353–355.
- Brown, R.C. Effect of Electric Charge in Filter Materials. Filtr. Sep. 1989, 26, 46–51.
- Scheuch, G. Breathing Is Enough: For the Spread of Influenza Virus and SARS-CoV-2 by Breathing Only. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 230–234.
- ISO 29463-1:2017(en)High Efficiency Filters and Filter Media for Removing Particles from Air—Part 1: Classification, Performance, Testing and Marking, ISO: Geneva, Switzerland, 2017; p. 26.
- ISO 16890-4:2016(en)Air Filters for General Ventilation—Part 4: Conditioning Method to Determine the Minimum Fractional Test Efficiency, ISO: Geneva, Switzerland, 2016; p. 20.
- EN779:2012(E)Particulate Air Filters for General Ventilation—Determination of the Filtration Performance, CEN: Brussels, Belgium, 2012; p. 74.
- Deng, X.; Gong, G.; He, X.; Shi, X.; Mo, L. Control of exhaled SARS-CoV-2-laden aerosols in the interpersonal breathing microenvironment in a ventilated room with limited space air stability. J. Environ. Sci. 2021, 108, 175–187.




