
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Learn-Han Lee | + 5415 word(s) | 5415 | 2022-01-04 07:00:42 | | | |
| 2 | Angel Yun-Kuan Thye | + 6 word(s) | 5421 | 2022-01-05 15:11:34 | | | | |
| 3 | Jodi Woan-Fei Law | + 9 word(s) | 5424 | 2022-01-05 17:46:18 | | |
Video Upload Options
There is growing evidence of studies associating COVID-19 survivors with increased mental health consequences. Mental health implications related to a COVID-19 infection include both acute and long-term consequences. Here we discuss COVID-19-associated psychiatric sequelae, particularly anxiety, depression, and post-traumatic stress disorder (PTSD), drawing parallels to past coronavirus outbreaks. A literature search was completed across three databases, using keywords to search for relevant articles. The cause may directly correlate to the infection through both direct and indirect mechanisms, but the underlying etiology appears more complex and multifactorial, involving environmental, psychological, and biological factors. Although most risk factors and prevalence rates vary across various studies, being of the female gender and having a history of psychiatric disorders seem consistent. Several studies will be presented, demonstrating COVID-19 survivors presenting higher rates of mental health consequences than the general population. The possible mechanisms by which the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) enters the brain, affecting the central nervous system (CNS) and causing these psychiatric sequelae, will be discussed, particularly concerning the SARS-CoV-2 entry via the angiotensin-converting enzyme 2 (ACE-2) receptors and the implications of the immune inflammatory signaling on neuropsychiatric disorders. Some possible therapeutic options will also be considered.
1. Introduction
2. Cause, Prevalence, and Risk Factors of COVID-19-Associated Psychological Effects
3. Psychiatric Sequelae of COVID-19
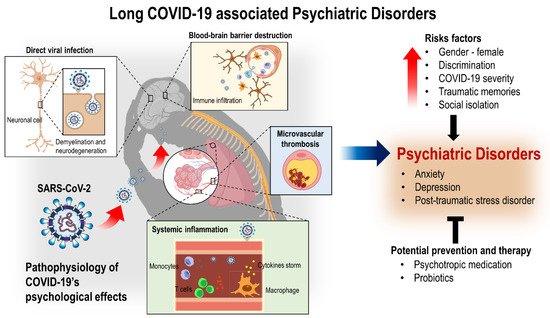
4. Pathophysiology of COVID-19’s Psychological Effects
4.1. SARS-CoV-2 Entry into the Brain/CNS
4.2. Implications of Immune Inflammatory Signaling on Neuropsychiatric Disorder
5. Possible Therapeutic Options
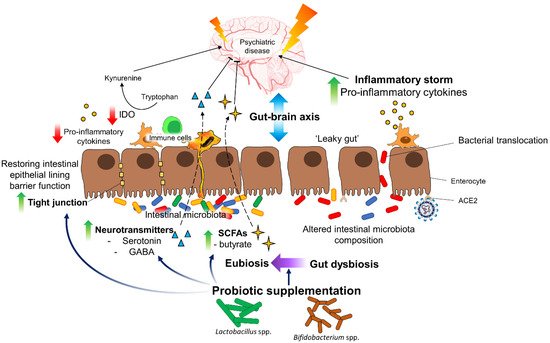
6. Conclusions
References
- Kumar, S.; Veldhuis, A.; Malhotra, T. Neuropsychiatric and Cognitive Sequelae of COVID-19. Front. Psychol. 2021, 12, 553.
- Mazza, M.G.; Palladini, M.; De Lorenzo, R.; Magnaghi, C.; Poletti, S.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; Benedetti, F.; COVID-19 BioB Outpatient Clinic Study group. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021, 94, 138–147.
- COVID-19 dashboard. Available online: https://coronavirus.jhu.edu/map.html (accessed on 19 October 2021).
- Thye, A.Y.-K.; Law, J.W.-F.; Pusparajah, P.; Letchumanan, V.; Chan, K.-G.; Lee, L.-H. Emerging SARS-CoV-2 variants of concern (VOCs): An impending global crisis. Biomedicines 2021, 9, 1303.
- Update on Omicron. Available online: https://www.who.int/news/item/28-11-2021-update-on-omicron (accessed on 5 December 2021).
- Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. Available online: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern (accessed on 5 December 2021).
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA 2020, 324, 782–793.
- Cevik, M.; Kuppalli, K.; Kindrachuk, J.; Peiris, M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ 2020, 371.
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232.
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615.
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574.
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154.
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Brüggen, M.C.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581.
- Haanen, C.; Vermes, I. Apoptosis and inflammation. Mediat. Inflamm. 1995, 4, 5–15.
- Yang, Y.; Jiang, G.; Zhang, P.; Fan, J. Programmed cell death and its role in inflammation. Mil. Med. Res. 2015, 2, 1–12.
- Nailwal, H.; Chan, F.K.-M. Necroptosis in anti-viral inflammation. Cell Death Differ. 2019, 26, 4–13.
- Jorgensen, I.; Miao, E.A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 2015, 265, 130–142.
- Zhou, X.; Ye, Q. Cellular Immune Response to COVID-19 and Potential Immune Modulators. Front. Immunol. 2021, 12, 1566.
- Levin, B.R.; Baquero, F.; Ankomah, P.P.; McCall, I.C. Phagocytes, antibiotics, and self-limiting bacterial infections. Trends Microbiol. 2017, 25, 878–892.
- Levin, B.R.; Antia, R. Why we don’t get sick: The within-host population dynamics of bacterial infections. Science 2001, 292, 1112–1115.
- Dobson, G.P.; Biros, E.; Letson, H.L.; Morris, J.L. Living in a Hostile World: Inflammation, New Drug Development, and Coronavirus. Front. Immunol. 2021, 11, 1–23.
- Icenogle, T. COVID-19: Infection or autoimmunity. Front. Immunol. 2020, 11, 1–11.
- Halpert, G.; Shoenfeld, Y. SARS-CoV-2, the autoimmune virus. Autoimmun. Rev. 2020, 19, 102695.
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Liu, C.; Yang, C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020, 87, 18–22.
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427.
- Xiang, Y.-T.; Yang, Y.; Li, W.; Zhang, L.; Zhang, Q.; Cheung, T.; Ng, C.H. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry 2020, 7, 228–229.
- Psychiatry, L. Send in the therapists? Lancet Psychiatry 2020, 7, 291.
- Amsalem, D.; Dixon, L.B.; Neria, Y. The coronavirus disease 2019 (COVID-19) outbreak and mental health: Current risks and recommended actions. JAMA Psychiatry 2021, 78, 9–10.
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627.
- Kępińska, A.P.; Iyegbe, C.O.; Vernon, A.C.; Yolken, R.; Murray, R.M.; Pollak, T.A. Schizophrenia and influenza at the centenary of the 1918-1919 Spanish influenza pandemic: Mechanisms of psychosis risk. Front. Psychiatry 2020, 11, 72.
- Ahmed, H.; Patel, K.; Greenwood, D.C.; Halpin, S.; Lewthwaite, P.; Salawu, A.; Eyre, L.; Breen, A.; O’Connor, R.; Jones, A. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J. Rehabil. Med. 2020, 52.
- Lam, M.H.-B.; Wing, Y.-K.; Yu, M.W.-M.; Leung, C.-M.; Ma, R.C.; Kong, A.P.; So, W.; Fong, S.Y.-Y.; Lam, S.-P. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: Long-term follow-up. Arch. Intern. Med. 2009, 169, 2142–2147.
- Hong, X.; Currier, G.W.; Zhao, X.; Jiang, Y.; Zhou, W.; Wei, J. Posttraumatic stress disorder in convalescent severe acute respiratory syndrome patients: A 4-year follow-up study. Gen. Hosp. Psychiatry 2009, 31, 546–554.
- Cheng, S.K.; Wong, C.; Tsang, J.; Wong, K. Psychological distress and negative appraisals in survivors of severe acute respiratory syndrome (SARS). Psychol. Med. 2004, 34, 1187–1195.
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594–600.
- Ma, Y.-F.; Li, W.; Deng, H.-B.; Wang, L.; Wang, Y.; Wang, P.-H.; Bo, H.-X.; Cao, J.; Wang, Y.; Zhu, L.-Y. Prevalence of depression and its association with quality of life in clinically stable patients with COVID-19. J. Affect. Disord. 2020, 275, 145–148.
- De Lorenzo, R.; Conte, C.; Lanzani, C.; Benedetti, F.; Roveri, L.; Mazza, M.G.; Brioni, E.; Giacalone, G.; Canti, V.; Sofia, V. Residual clinical damage after COVID-19: A retrospective and prospective observational cohort study. PLoS ONE 2020, 15, e0239570.
- Varatharaj, A.; Thomas, N.; Ellul, M.A.; Davies, N.W.; Pollak, T.A.; Tenorio, E.L.; Sultan, M.; Easton, A.; Breen, G.; Zandi, M. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry 2020, 7, 875–882.
- Siu, J.Y.-M. The SARS-associated stigma of SARS victims in the post-SARS era of Hong Kong. Qual. Health Res. 2008, 18, 729–738.
- Jones, C.; Humphris, G.; Griffiths, R. Psychological morbidity following critical illness-the rationale for care after intensive care. Clin. Intensive Care 1998, 9, 199–205.
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet 2020, 395, 912–920.
- Asmundson, G.J.; Taylor, S. Coronaphobia: Fear and the 2019-nCoV outbreak. J. Anxiety Disord. 2020, 70, 102196.
- Chaves, C.; Castellanos, T.; Abrams, M.; Vazquez, C. The impact of economic recessions on depression and individual and social well-being: The case of Spain (2006–2013). Soc. Psychiatry Psychiatr. Epidemiol. 2018, 53, 977–986.
- Greenberg, N.; Docherty, M.; Gnanapragasam, S.; Wessely, S. Managing mental health challenges faced by healthcare workers during covid-19 pandemic. BMJ 2020, 368.
- Vanderlind, W.M.; Rabinovitz, B.B.; Miao, I.Y.; Oberlin, L.E.; Bueno-Castellano, C.; Fridman, C.; Jaywant, A.; Kanellopoulos, D. A systematic review of neuropsychological and psychiatric sequalae of COVID-19: Implications for treatment. Curr. Opin. Psychiatry 2021, 34, 420.
- De Sousa, G.M.; Júnior, V.D.d.O.T.; de Meiroz Grilo, M.L.P.; Coelho, M.L.G.; de Lima-Araújo, G.L.; Schuch, F.B.; Galvão-Coelho, N.L. Mental Health in COVID-19 Pandemic: A Meta-Review of Prevalence Meta-Analyses. Front. Psychol. 2021, 12.
- Nie, X.-D.; Wang, Q.; Wang, M.-N.; Zhao, S.; Liu, L.; Zhu, Y.-L.; Chen, H. Anxiety and depression and its correlates in patients with coronavirus disease 2019 in Wuhan. Int. J. Psychiatry Clin. Pract. 2021, 25, 109–114.
- Shanbehzadeh, S.; Tavahomi, M.; Zanjari, N.; Ebrahimi-Takamjani, I.; Amiri-Arimi, S. Physical and mental health complications post-COVID-19: Scoping review. J. Psychosom. Res. 2021, 110525.
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 symptom burden: What is long-COVID and how should we manage it? Lung 2021, 199, 113–119.
- Raman, B.; Cassar, M.P.; Tunnicliffe, E.M.; Filippini, N.; Griffanti, L.; Alfaro-Almagro, F.; Okell, T.; Sheerin, F.; Xie, C.; Mahmod, M. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine 2021, 31, 100683.
- Tomasoni, D.; Bai, F.; Castoldi, R.; Barbanotti, D.; Falcinella, C.; Mulè, G.; Mondatore, D.; Tavelli, A.; Vegni, E.; Marchetti, G. Anxiety and depression symptoms after virological clearance of COVID-19: A cross-sectional study in Milan, Italy. J. Med. Virol. 2021, 93, 1175–1179.
- Islam, M.; Islam, U.S.; Mosaddek, A.S.M.; Potenza, M.N.; Pardhan, S. Treatment, persistent symptoms, and depression in people infected with COVID-19 in Bangladesh. Int. J. Environ. Res. Public Health 2021, 18, 1453.
- Van den Borst, B.; Peters, J.B.; Brink, M.; Schoon, Y.; Bleeker-Rovers, C.P.; Schers, H.; van Hees, H.W.; van Helvoort, H.; van den Boogaard, M.; van der Hoeven, H. Comprehensive health assessment three months after recovery from acute COVID-19. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020.
- De Graaf, M.; Antoni, M.; Ter Kuile, M.; Arbous, M.; Duinisveld, A.; Feltkamp, M.; Groeneveld, G.; Hinnen, S.; Janssen, V.; Lijfering, W. Short-term outpatient follow-up of COVID-19 patients: A multidisciplinary approach. EClinicalMedicine 2021, 32, 100731.
- Bonazza, F.; Borghi, L.; di San Marco, E.C.; Piscopo, K.; Bai, F.; Monforte, A.d.A.; Vegni, E. Psychological outcomes after hospitalization for COVID-19: Data from a multidisciplinary follow-up screening program for recovered patients. Res. Psychother. Psychopathol. Process Outcome 2020, 23, 247–255.
- Bellan, M.; Soddu, D.; Balbo, P.E.; Baricich, A.; Zeppegno, P.; Avanzi, G.C.; Baldon, G.; Bartolomei, G.; Battaglia, M.; Battistini, S. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw. Open 2021, 4, e2036142.
- Tripathy, S.; Acharya, S.P.; Singh, S.; Patra, S.; Mishra, B.R.; Kar, N. Post traumatic stress symptoms, anxiety, and depression in patients after intensive care unit discharge–a longitudinal cohort study from a LMIC tertiary care centre. BMC Psychiatry 2020, 20, 1–11.
- Wang, C.; Chudzicka-Czupała, A.; Tee, M.L.; Núñez, M.I.L.; Tripp, C.; Fardin, M.A.; Habib, H.A.; Tran, B.X.; Adamus, K.; Anlacan, J. A chain mediation model on COVID-19 symptoms and mental health outcomes in Americans, Asians and Europeans. Sci. Rep. 2021, 11, 1–12.
- Xiong, J.; Lipsitz, O.; Nasri, F.; Lui, L.M.; Gill, H.; Phan, L.; Chen-Li, D.; Iacobucci, M.; Ho, R.; Majeed, A. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J. Affect. Disord. 2020.
- Liu, D.; Baumeister, R.F.; Veilleux, J.C.; Chen, C.; Liu, W.; Yue, Y.; Zhang, S. Risk factors associated with mental illness in hospital discharged patients infected with COVID-19 in Wuhan, China. Psychiatry Res. 2020, 292, 113297.
- Mannan, A.; Mehedi, H.; Chy, N.; Qayum, M.O.; Akter, F.; Rob, M.; Biswas, P.; Hossain, S.; Ayub, M.I. A multi-centre, cross-sectional study on coronavirus disease 2019 in Bangladesh: Clinical epidemiology and short-term outcomes in recovered individuals. New Microbes New Infect. 2021, 40, 100838.
- Cai, X.; Hu, X.; Ekumi, I.O.; Wang, J.; An, Y.; Li, Z.; Yuan, B. Psychological distress and its correlates among COVID-19 survivors during early convalescence across age groups. Am. J. Geriatr. Psychiatry 2020, 28, 1030–1039.
- Speth, M.M.; Singer-Cornelius, T.; Oberle, M.; Gengler, I.; Brockmeier, S.J.; Sedaghat, A.R. Mood, anxiety and olfactory dysfunction in COVID-19: Evidence of central nervous system involvement? Laryngoscope 2020, 130, 2520–2525.
- Wang, P.R.; Oyem, P.C.; Viguera, A.C. Prevalence of psychiatric morbidity following discharge after COVID-19 hospitalization. Gen. Hosp. Psychiatry 2020, 69, 131–132.
- Kang, E.; Lee, S.Y.; Kim, M.S.; Jung, H.; Kim, K.H.; Kim, K.-N.; Park, H.Y.; Lee, Y.J.; Cho, B.; Sohn, J.H. The psychological burden of COVID-19 stigma: Evaluation of the mental health of isolated mild condition COVID-19 patients. J. Korean Med. Sci. 2021, 36.
- Hajure, M.; Tariku, M.; Mohammedhussein, M.; Dule, A. Depression, Anxiety and Associated Factors Among Chronic Medical Patients Amid COVID-19 Pandemic in Mettu Karl Referral Hospital, Mettu, Ethiopia, 2020. Neuropsychiatr. Dis. Treat. 2020, 16, 2511–2518.
- Almeria, M.; Cejudo, J.C.; Sotoca, J.; Deus, J.; Krupinski, J. Cognitive profile following COVID-19 infection: Clinical predictors leading to neuropsychological impairment. Brain Behav. Immun. Health 2020, 9, 100163.
- Baroiu, L.; Dumea, E.; Nastase, F.; Niculet, E.; Fotea, S.; Ciubara, A.B.; Stefanopol, I.A.; Nechita, A.; Anghel, L.T.; Ciubara, A. Assessment of Depression in Patients with COVID-19. Brain-Broad Res. Artif. Intell. Neurosci. 2021, 12, 254–264.
- Yuan, B.; Li, W.; Liu, H.; Cai, X.; Song, S.; Zhao, J.; Hu, X.; Li, Z.; Chen, Y.; Zhang, K. Correlation between immune response and self-reported depression during convalescence from COVID-19. Brain Behav. Immun. 2020, 88, 39–43.
- Mandal, S.; Barnett, J.; Brill, S.E.; Brown, J.S.; Denneny, E.K.; Hare, S.S.; Heightman, M.; Hillman, T.E.; Jacob, J.; Jarvis, H.C. ‘Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021, 76, 396–398.
- Park, H.Y.; Jung, J.; Park, H.Y.; Lee, S.H.; Kim, E.S.; Kim, H.B.; Song, K.-H. Psychological consequences of survivors of COVID-19 pneumonia 1 month after discharge. J. Korean Med. Sci. 2020, 35.
- Matalon, N.; Dorman-Ilan, S.; Hasson-Ohayon, I.; Hertz-Palmor, N.; Shani, S.; Basel, D.; Gross, R.; Chen, W.; Abramovich, A.; Afek, A.; et al. Trajectories of post-traumatic stress symptoms, anxiety, and depression in hospitalized COVID-19 patients: A one-month follow-up. J. Psychosom. Res. 2021, 143, 4.
- Chen, Y.; Huang, X.; Zhang, C.; An, Y.; Liang, Y.; Yang, Y.; Liu, Z. Prevalence and predictors of posttraumatic stress disorder, depression and anxiety among hospitalized patients with coronavirus disease 2019 in China. BMC Psychiatry 2021, 21, 1–8.
- Vindegaard, N.; Benros, M.E. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav. Immun. 2020, 89, 531–542.
- Ozamiz-Etxebarria, N.; Dosil-Santamaria, M.; Picaza-Gorrochategui, M.; Idoiaga-Mondragon, N. Stress, anxiety, and depression levels in the initial stage of the COVID-19 outbreak in a population sample in the northern Spain. Cad. Saude Publica 2020, 36.
- Pappa, S.; Ntella, V.; Giannakas, T.; Giannakoulis, V.G.; Papoutsi, E.; Katsaounou, P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: A systematic review and meta-analysis. Brain Behav. Immun. 2020, 88, 901–907.
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J. Med. Virol. 2021, 93, 1013–1022.
- Mendez, R.; Balanzá-Martínez, V.; Luperdi, S.C.; Estrada, I.; Latorre, A.; González-Jiménez, P.; Feced, L.; Bouzas, L.; Yepez, K.; Ferrando, A. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J. Intern. Med. 2021.
- Chen, K.-Y.; Li, T.; Gong, F.; Zhang, J.-S.; Li, X.-K. Predictors of health-related quality of life and influencing factors for COVID-19 patients, a follow-up at one month. Front. Psychiatry 2020, 11, 668.
- Lee, A.M.; Wong, J.G.; McAlonan, G.M.; Cheung, V.; Cheung, C.; Sham, P.C.; Chu, C.-M.; Wong, P.-C.; Tsang, K.W.; Chua, S.E. Stress and psychological distress among SARS survivors 1 year after the outbreak. Can. J. Psychiatry 2007, 52, 233–240.
- Lega, I.; Nistico, L.; Palmieri, L.; Caroppo, E.; Lo Noce, C.; Donfrancesco, C.; Vanacore, N.; Scattoni, M.L.; Picardi, A.; Gigantesco, A.; et al. Psychiatric disorders among hospitalized patients deceased with COVID-19 in Italy. EClinicalMedicine 2021, 35, 7.
- Li, X.Y.; Tian, J.; Xu, Q. The Associated Factors of Anxiety and Depressive Symptoms in COVID-19 Patients Hospitalized in Wuhan, China. Psychiatric Quarterly 2021, 92, 879–887.
- Daher, A.; Balfanz, P.; Cornelissen, C.; Müller, A.; Bergs, I.; Marx, N.; Müller-Wieland, D.; Hartmann, B.; Dreher, M.; Müller, T. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir. Med. 2020, 174, 106197.
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann. Clin. Transl. Neurol 2021, 8, 1073–1085.
- Postolache, T.T.; Benros, M.E.; Brenner, L.A. Targetable biological mechanisms implicated in emergent psychiatric conditions associated with SARS-CoV-2 infection. JAMA Psychiatry 2021, 78, 353–354.
- Tansey, C.M.; Louie, M.; Loeb, M.; Gold, W.L.; Muller, M.P.; de Jager, J.; Cameron, J.I.; Tomlinson, G.; Mazzulli, T.; Walmsley, S.L. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch. Intern. Med. 2007, 167, 1312–1320.
- Vincent, A.; Beck, K.; Becker, C.; Zumbrunn, S.; Ramin-Wright, M.; Urben, T.; Quinto, A.; Schaefert, R.; Meinlschmidt, G.; Gaab, J.; et al. Psychological burden in patients with COVID-19 and their relatives 90 days after hospitalization: A prospective observational cohort study. J. Psychosom. Res. 2021, 147, 9.
- Schandl, A.; Hedman, A.; Lyng, P.; Tachinabad, S.F.; Svefors, J.; Rol, M.; Geborek, A.; Franko, M.A.; Sderberg, M.; Joelsson-Alm, E.; et al. Long-term consequences in critically ill COVID-19 patients: A prospective cohort study. Acta Anaesthesiol. Scand. 2021, 65, 1285–1292.
- Horwitz, L.I.; Garry, K.; Prete, A.M.; Sharma, S.; Mendoza, F.; Kahan, T.; Karpel, H.; Duan, E.; Hochman, K.A.; Weerahandi, H. Six-Month Outcomes in Patients Hospitalized with Severe COVID-19. J. Gen. Intern. Med. 2021, 36, 3772–3777.
- Frontera, J.A.; Yang, D.; Lewis, A.; Patel, P.; Medicherla, C.; Arena, V.; Fang, T.; Andino, A.; Snyder, T.; Madhavan, M.; et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J. Neurol. Sci. 2021, 426, 117486.
- Latronico, N.; Peli, E.; Calza, S.; Rodella, F.; Novelli, M.P.; Cella, A.; Marshall, J.; Needham, D.M.; Rasulo, F.A.; Piva, S.; et al. Physical, cognitive and mental health outcomes in 1-year survivors of COVID-19-associated ARDS. Thorax 2021, 4.
- Becker, C.; Beck, K.; Zumbrunn, S.; Memma, V.; Herzog, N.; Bissmann, B.; Gross, S.; Loretz, N.; Mueller, J.; Amacher, S.A.; et al. Long COVID 1 year after hospitalisation for COVID-19: A prospective bicentric cohort study. Swiss Med. Wkly. 2021, 151, w30091.
- Altman, M.T.; Knauert, M.P.; Pisani, M.A. Sleep disturbance after hospitalization and critical illness: A systematic review. Ann. Am. Thorac. Soc. 2017, 14, 1457–1468.
- Phiri, P.; Ramakrishnan, R.; Rathod, S.; Elliot, K.; Thayanandan, T.; Sandle, N.; Haque, N.; Chau, S.W.; Wong, O.W.; Chan, S.S. An evaluation of the mental health impact of SARS-CoV-2 on patients, general public and healthcare professionals: A systematic review and meta-analysis. EClinicalMedicine 2021, 34, 100806.
- Janiri, D.; Kotzalidis, G.D.; Giuseppin, G.; Molinaro, M.; Modica, M.; Montanari, S.; Terenzi, B.; Carfì, A.; Landi, F.; Sani, G. Psychological distress after Covid-19 recovery: Reciprocal effects with temperament and emotional dysregulation. An exploratory study of patients over 60 years of age assessed in a post-acute care service. Front. Psychiatry 2020, 11, 1210.
- Guo, L.; Lin, J.; Ying, W.; Zheng, C.; Tao, L.; Ying, B.; Cheng, B.; Jin, S.; Hu, B. Correlation study of short-term mental health in patients discharged after coronavirus disease 2019 (COVID-19) infection without comorbidities: A prospective study. Neuropsychiatr. Dis. Treat. 2020, 16, 2661.
- Garrigues, E.; Janvier, P.; Kherabi, Y.; Le Bot, A.; Hamon, A.; Gouze, H.; Doucet, L.; Berkani, S.; Oliosi, E.; Mallart, E. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020, 81, e4–e6.
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427.
- Huang, L.X.; Yao, Q.; Gu, X.Y.; Wang, Q.Y.; Ren, L.L.; Wang, Y.M.; Hu, P.; Guo, L.; Liu, M.; Xu, J.Y.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758.
- Frontera, J.A.; Lewis, A.; Melmed, K.; Lin, J.; Kondziella, D.; Helbok, R.; Yaghi, S.; Meropol, S.; Wisniewski, T.; Balcer, L. Prevalence and Predictors of Prolonged Cognitive and Psychological Symptoms Following COVID-19 in the United States. Front. Aging Neurosci. 2021, 357.
- Carfì, A.; Bernabei, R.; Landi, F. Persistent symptoms in patients after acute COVID-19. JAMA 2020, 324, 603–605.
- Tenforde, M.W. Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged≥ 65 Years—United States, January–March 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70.
- Mahmud, R.; Rahman, M.M.; Rassel, M.A.; Monayem, F.B.; Sayeed, S.J.B.; Islam, M.S.; Islam, M.M. Post-COVID-19 syndrome among symptomatic COVID-19 patients: A prospective cohort study in a tertiary care center of Bangladesh. PLoS ONE 2021, 16, e0249644.
- El Sayed, S.; Shokry, D.; Gomaa, S.M. Post-COVID-19 fatigue and anhedonia: A cross-sectional study and their correlation to post-recovery period. Neuropsychopharmacol. Rep. 2021, 41, 50–55.
- Mak, I.W.C.; Chu, C.M.; Pan, P.C.; Yiu, M.G.C.; Chan, V.L. Long-term psychiatric morbidities among SARS survivors. Gen. Hosp. Psychiatry 2009, 31, 318–326.
- Robinson, E.; Sutin, A.R.; Daly, M.; Jones, A. A systematic review and meta-analysis of longitudinal cohort studies comparing mental health before versus during the COVID-19 pandemic in 2020. J. Affect. Disord. 2022, 296, 567–576.
- Arabi, Y.; Harthi, A.; Hussein, J.; Bouchama, A.; Johani, S.; Hajeer, A.; Saeed, B.; Wahbi, A.; Saedy, A.; AlDabbagh, T. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV). Infection 2015, 43, 495–501.
- Gu, J.; Gong, E.; Zhang, B.; Zheng, J.; Gao, Z.; Zhong, Y.; Zou, W.; Zhan, J.; Wang, S.; Xie, Z. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005, 202, 415–424.
- Xu, J.; Zhong, S.; Liu, J.; Li, L.; Li, Y.; Wu, X.; Li, Z.; Deng, P.; Zhang, J.; Zhong, N. Detection of severe acute respiratory syndrome coronavirus in the brain: Potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2005, 41, 1089–1096.
- Lee, S.H.; Shin, H.-S.; Park, H.Y.; Kim, J.L.; Lee, J.J.; Lee, H.; Won, S.-D.; Han, W. Depression as a mediator of chronic fatigue and post-traumatic stress symptoms in Middle East respiratory syndrome survivors. Psychiatry Investig. 2019, 16, 59.
- Arab-Zozani, M.; Hashemi, F.; Safari, H.; Yousefi, M.; Ameri, H. Health-related quality of life and its associated factors in COVID-19 patients. Osong Public Health Res. Perspect. 2020, 11, 296.
- Kim, Y.; Kim, S.W.; Chang, H.H.; Kwon, K.T.; Bae, S.; Hwang, S. Significance and Associated Factors of Long-Term Sequelae in Patients after Acute COVID-19 Infection in Korea. Infect. Chemother. 2021, 53, 463–476.
- Taquet, M.; Luciano, S.; Geddes, J.R.; Harrison, P.J. Bidirectional associations between COVID-19 and psychiatric disorder: Retrospective cohort studies of 62 354 COVID-19 cases in the USA. The Lancet Psychiatry 2021, 8, 130–140.
- Bo, H.-X.; Li, W.; Yang, Y.; Wang, Y.; Zhang, Q.; Cheung, T.; Wu, X.; Xiang, Y.-T. Posttraumatic stress symptoms and attitude toward crisis mental health services among clinically stable patients with COVID-19 in China. Psychol. Med. 2021, 51, 1052–1053.
- Guo, Q.; Zheng, Y.; Shi, J.; Wang, J.; Li, G.; Li, C.; Fromson, J.A.; Xu, Y.; Liu, X.; Xu, H. Immediate psychological distress in quarantined patients with COVID-19 and its association with peripheral inflammation: A mixed-method study. Brain Behav. Immun. 2020, 88, 17–27.
- Liu, D.; Wang, Y.; Wang, J.; Liu, J.; Yue, Y.; Liu, W.; Zhang, F.; Wang, Z. Characteristics and outcomes of a sample of patients with COVID-19 identified through social media in Wuhan, China: Observational study. J. Med. Internet Res. 2020, 22, e20108.
- Huang, Y.; Wang, Y.; Wang, H.; Liu, Z.; Yu, X.; Yan, J.; Yu, Y.; Kou, C.; Xu, X.; Lu, J. Prevalence of mental disorders in China: A cross-sectional epidemiological study. Lancet Psychiatry 2019, 6, 211–224.
- Bohmwald, K.; Galvez, N.; Ríos, M.; Kalergis, A.M. Neurologic alterations due to respiratory virus infections. Front. Cell. Neurosci. 2018, 12, 386.
- Zhou, Z.; Kang, H.; Li, S.; Zhao, X. Understanding the neurotropic characteristics of SARS-CoV-2: From neurological manifestations of COVID-19 to potential neurotropic mechanisms. J. Neurol. 2020, 267, 2179–2184.
- Romero-Sánchez, C.M.; Díaz-Maroto, I.; Fernández-Díaz, E.; Sánchez-Larsen, Á.; Layos-Romero, A.; García-García, J.; González, E.; Redondo-Peñas, I.; Perona-Moratalla, A.B.; Del Valle-Pérez, J.A. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology 2020, 95, e1060–e1070.
- Reichard, R.R.; Kashani, K.B.; Boire, N.A.; Constantopoulos, E.; Guo, Y.; Lucchinetti, C.F. Neuropathology of COVID-19: A spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020, 140, 1–6.
- MacIntosh, B.J.; Ji, X.; Chen, J.J.; Gilboa, A.; Roudaia, E.; Sekuler, A.B.; Gao, F.; Chad, J.A.; Jegatheesan, A.; Masellis, M.; et al. Brain structure and function in people recovering from COVID-19 after hospital discharge or self-isolation: A longitudinal observational study protocol. CMAJ Open 2021, 9, E1114–E1119.
- Heneka, M.T.; Golenbock, D.; Latz, E.; Morgan, D.; Brown, R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res. Ther. 2020, 12, 1–3.
- Muccioli, L.; Pensato, U.; Cani, I.; Guarino, M.; Cortelli, P.; Bisulli, F. COVID-19-associated encephalopathy and cytokine-mediated neuroinflammation. Ann. Neurol. 2020, 88, 860–861.
- Pilotto, A.; Padovani, A. Reply to the Letter “COVID-19-Associated Encephalopathy and Cytokine-Mediated Neuroinflammation”. Ann. Neurol. 2020, 88, 861–862.
- South, K.; McCulloch, L.; McColl, B.W.; Elkind, M.S.; Allan, S.M.; Smith, C.J. Preceding infection and risk of stroke: An old concept revived by the COVID-19 pandemic. Int. J. Stroke 2020, 15, 722–732.
- Bechter, K. Virus infection as a cause of inflammation in psychiatric disorders. Inflamm. Psychiatry 2013, 28, 49–60.
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 1–8.
- Xia, H.; Lazartigues, E. Angiotensin-converting enzyme 2 in the brain: Properties and future directions. J. Neurochem. 2008, 107, 1482–1494.
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418.
- Lippi, A.; Domingues, R.; Setz, C.; Outeiro, T.F.; Krisko, A. SARS-CoV-2: At the crossroad between aging and neurodegeneration. Mov. Disord. 2020, 35, 716.
- Bortolato, B.; Carvalho, A.F.; Soczynska, J.K.; Perini, G.I.; McIntyre, R.S. The involvement of TNF-α in cognitive dysfunction associated with major depressive disorder: An opportunity for domain specific treatments. Curr. Neuropharmacol. 2015, 13, 558–576.
- Subramaniyan, S.; Terrando, N. Narrative Review Article: Neuroinflammation and Perioperative Neurocognitive Disorders. Anesth. Analg. 2019, 128, 781–788.
- Wang, R.P.-H.; Ho, Y.-S.; Leung, W.K.; Goto, T.; Chang, R.C.-C. Systemic inflammation linking chronic periodontitis to cognitive decline. Brain Behav. Immun. 2019, 81, 63–73.
- Dantzer, R. Neuroimmune interactions: From the brain to the immune system and vice versa. Physiol. Rev. 2018, 98, 477–504.
- Troyer, E.A.; Kohn, J.N.; Hong, S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020, 87, 34–39.
- Köhler, C.A.; Freitas, T.H.; Maes, M.d.; De Andrade, N.; Liu, C.; Fernandes, B.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387.
- Miller, B.J.; Buckley, P.; Seabolt, W.; Mellor, A.; Kirkpatrick, B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol. Psychiatry 2011, 70, 663–671.
- Renna, M.E.; O’Toole, M.S.; Spaeth, P.E.; Lekander, M.; Mennin, D.S. The association between anxiety, traumatic stress, and obsessive–compulsive disorders and chronic inflammation: A systematic review and meta-analysis. Depress. Anxiety 2018, 35, 1081–1094.
- Poletti, S.; Leone, G.; Hoogenboezem, T.A.; Ghiglino, D.; Vai, B.; de Wit, H.; Wijkhuijs, A.J.; Locatelli, C.; Colombo, C.; Drexhage, H.A. Markers of neuroinflammation influence measures of cortical thickness in bipolar depression. Psychiatry Res. Neuroimaging 2019, 285, 64–66.
- Benedetti, F.; Aggio, V.; Pratesi, M.L.; Greco, G.; Furlan, R. Neuroinflammation in bipolar depression. Front. Psychiatry 2020, 11, 71.
- Benedetti, F.; Poletti, S.; Hoogenboezem, T.A.; Locatelli, C.; de Wit, H.; Wijkhuijs, A.J.; Colombo, C.; Drexhage, H.A. Higher baseline proinflammatory cytokines mark poor antidepressant response in bipolar disorder. J. Clin. Psychiatr. 2017, 78, e986–e993.
- Najjar, S.; Pearlman, D.M.; Alper, K.; Najjar, A.; Devinsky, O. Neuroinflammation and psychiatric illness. J. Neuroinflamm. 2013, 10, 1–24.
- Jones, K.A.; Thomsen, C. The role of the innate immune system in psychiatric disorders. Mol. Cell. Neurosci. 2013, 53, 52–62.
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34.
- Cameron, M.J.; Bermejo-Martin, J.F.; Danesh, A.; Muller, M.P.; Kelvin, D.J. Human immunopathogenesis of severe acute respiratory syndrome (SARS). Virus Res. 2008, 133, 13–19.
- Yuan, N.; Chen, Y.; Xia, Y.; Dai, J.; Liu, C. Inflammation-related biomarkers in major psychiatric disorders: A cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl. Psychiatry 2019, 9, 1–13.
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32.
- Aziz, M.; Fatima, R.; Assaly, R. Elevated interleukin-6 and severe COVID-19: A meta-analysis. J. Med. Virol. 2020.
- Pairo-Castineira, E.; Clohisey, S.; Klaric, L.; Bretherick, A.D.; Rawlik, K.; Pasko, D.; Walker, S.; Parkinson, N.; Fourman, M.H.; Russell, C.D. Genetic mechanisms of critical illness in Covid-19. Nature 2021, 591, 92–98.
- Zhu, J.; Pang, J.; Ji, P.; Zhong, Z.; Li, H.; Li, B.; Zhang, J. Elevated interleukin-6 is associated with severity of COVID-19: A meta-analysis. J. Med. Virol. 2020.
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020, 8, 1233–1244.
- Wohleb, E.S.; Franklin, T.; Iwata, M.; Duman, R.S. Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci. 2016, 17, 497–511.
- Poletti, S.; Vai, B.; Mazza, M.G.; Zanardi, R.; Lorenzi, C.; Calesella, F.; Cazzetta, S.; Branchi, I.; Colombo, C.; Furlan, R. A peripheral inflammatory signature discriminates bipolar from unipolar depression: A machine learning approach. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 105, 110136.
- Gibney, S.M.; Drexhage, H.A. Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J. Neuroimmune Pharmacol. 2013, 8, 900–920.
- Grosse, L.; Carvalho, L.A.; Wijkhuijs, A.J.; Bellingrath, S.; Ruland, T.; Ambrée, O.; Alferink, J.; Ehring, T.; Drexhage, H.A.; Arolt, V. Clinical characteristics of inflammation-associated depression: Monocyte gene expression is age-related in major depressive disorder. Brain Behav. Immun. 2015, 44, 48–56.
- Frontera, J.A.; Sabadia, S.; Lalchan, R.; Fang, T.; Flusty, B.; Millar-Vernetti, P.; Snyder, T.; Berger, S.; Yang, D.; Granger, A. A prospective study of neurologic disorders in hospitalized patients with COVID-19 in New York City. Neurology 2021, 96, e575–e586.
- Frontera, J.A.; Valdes, E.; Huang, J.; Lewis, A.; Lord, A.S.; Zhou, T.; Kahn, D.E.; Melmed, K.; Czeisler, B.M.; Yaghi, S. Prevalence and impact of hyponatremia in patients with coronavirus disease 2019 in New York City. Crit. Care Med. 2020.
- Drevets, W.C.; Savitz, J.; Trimble, M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008, 13, 663.
- Harrison, N.A.; Brydon, L.; Walker, C.; Gray, M.A.; Steptoe, A.; Critchley, H.D. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatry 2009, 66, 407–414.
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186.
- Enache, D.; Pariante, C.M.; Mondelli, V. Markers of central inflammation in major depressive disorder: A systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav. Immun. 2019, 81, 24–40.
- Eyre, H.A.; Air, T.; Pradhan, A.; Johnston, J.; Lavretsky, H.; Stuart, M.J.; Baune, B.T. A meta-analysis of chemokines in major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 68, 1–8.
- Huang, H.; Liu, Q.; Zhu, L.; Zhang, Y.; Lu, X.; Wu, Y.; Liu, L. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci. Rep. 2019, 9, 1–9.
- Johnson, D.; Thurairajasingam, S.; Letchumanan, V.; Chan, K.-G.; Lee, L.-H. Exploring the Role and Potential of Probiotics in the Field of Mental Health: Major Depressive Disorder. Nutrients 2021, 13, 1728.
- Liu, R.T.; Walsh, R.F.; Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23.
- De Araújo, F.F.; de Paulo Farias, D. Psychobiotics: An emerging alternative to ensure mental health amid the COVID-19 outbreak? Trends Food Sci. Technol. 2020, 103, 386.
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 2016, 6, e939.
- Olaimat, A.N.; Aolymat, I.; Al-Holy, M.; Ayyash, M.; Ghoush, M.A.; Al-Nabulsi, A.A.; Osaili, T.; Apostolopoulos, V.; Liu, S.-Q.; Shah, N.P. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. npj Sci. Food 2020, 4, 1–7.
- Büttiker, P.; Weissenberger, S.; Stefano, G.B.; Kream, R.M.; Ptacek, R. SARS-CoV-2, Trait Anxiety, and the Microbiome. Front. Psychiatry 2021, 12.
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A.D. Probiotics, prebiotics and immunomodulation of gut mucosal defences: Homeostasis and immunopathology. Nutrients 2013, 5, 1869–1912.
- Gohil, K.; Samson, R.; Dastager, S.; Dharne, M. Probiotics in the prophylaxis of COVID-19: Something is better than nothing. 3 Biotech 2021, 11, 1–10.
- Lehtoranta, L.; Pitkäranta, A.; Korpela, R. Probiotics in respiratory virus infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1289–1302.
- Purton, T.; Staskova, L.; Lane, M.M.; Dawson, S.L.; West, M.; Firth, J.; Clarke, G.; Cryan, J.F.; Berk, M.; O’Neil, A. Prebiotic and probiotic supplementation and the tryptophan-kynurenine pathway: A systematic review and meta analysis. Neurosci. Biobehav. Rev. 2021.
- Raison, C.L.; Rook, G.W.; Miller, A.H.; Begay, T.K. Role of inflammation in psychiatric disease. In Neurobiology of Brain Disorders; Elsevier: Amsterdam, The Netherlands, 2015; pp. 396–421.
- Yamawaki, Y.; Yoshioka, N.; Nozaki, K.; Ito, H.; Oda, K.; Harada, K.; Shirawachi, S.; Asano, S.; Aizawa, H.; Yamawaki, S. Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain Res. 2018, 1680, 13–38.
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, ra158–ra263.
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health benefits of Lactobacillus gasseri CP2305 tablets in young adults exposed to chronic stress: A randomized, double-blind, placebo-controlled study. Nutrients 2019, 11, 1859.
- Sawada, D.; Kuwano, Y.; Tanaka, H.; Hara, S.; Uchiyama, Y.; Sugawara, T.; Fujiwara, S.; Rokutan, K.; Nishida, K. Daily intake of Lactobacillus gasseri CP2305 relieves fatigue and stress-related symptoms in male university Ekiden runners: A double-blind, randomized, and placebo-controlled clinical trial. J. Funct. Foods 2019, 57, 465–476.
- Letchumanan, V.; Thye, A.Y.-K.; Tan, L.T.-H.; Law, J.W.-F.; Johnson, D.; Ser, H.-L.; Bhuvanendran, S.; Thurairajasingam, S.; Lee, L.-H. Gut feelings in depression: Microbiota dysbiosis in response to antidepressants. Gut 2021, A49–A50.
- Tabacof, L.; Tosto-Mancuso, J.; Wood, J.; Cortes, M.; Kontorovich, A.; McCarthy, D.; Rizk, D.; Rozanski, G.; Breyman, E.; Nasr, L.; et al. Post-acute COVID-19 syndrome negatively impacts physical function, cognitive function, health-related quality of life and participation. Am. J. Phys. Med. Rehabil. 2021, 101, 48–52.
- Bottemanne, H.; Gouraud, C.; Hulot, J.S.; Blanchard, A.; Ranque, B.; Lahlou-Laforêt, K.; Limosin, F.; Günther, S.; Lebeaux, D.; Lemogne, C. Do Anxiety and Depression Predict Persistent Physical Symptoms After a Severe COVID-19 Episode? A Prospective Study. Front. Psychiatry 2021, 12, 757685.
- Bonizzato, S.; Ghiggia, A.; Ferraro, F.; Galante, E. Cognitive, behavioral, and psychological manifestations of COVID-19 in post-acute rehabilitation setting: Preliminary data of an observational study. Neurol. Sci. 2021, 1–8.
- Gouraud, C.; Bottemanne, H.; Lahlou-Laforêt, K.; Blanchard, A.; Günther, S.; Batti, S.E.; Auclin, E.; Limosin, F.; Hulot, J.S.; Lebeaux, D.; et al. Association Between Psychological Distress, Cognitive Complaints, and Neuropsychological Status After a Severe COVID-19 Episode: A Cross-Sectional Study. Front Psychiatry 2021, 12, 725861.
- Poletti, S.; Palladini, M.; Mazza, M.G.; De Lorenzo, R.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; Benedetti, F.; Covid- Biob Outpatient Clinic, S. Long-term consequences of COVID-19 on cognitive functioning up to 6 months after discharge: Role of depression and impact on quality of life. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 1–10.
- Boesl, F.; Audebert, H.; Endres, M.; Pruss, H.; Franke, C. A Neurological Outpatient Clinic for Patients With Post-COVID-19 Syndrome—A Report on the Clinical Presentations of the First 100 Patients. Front. Neurol. 2021, 12.
- Mahmoudi, H.; Saffari, M.; Movahedi, M.; Sanaeinasab, H.; Rashidi-Jahan, H.; Pourgholami, M.; Poorebrahim, A.; Barshan, J.; Ghiami, M.; Khoshmanesh, S.; et al. A mediating role for mental health in associations between COVID-19-related self-stigma, PTSD, quality of life, and insomnia among patients recovered from COVID-19. Brain Behav 2021, 11, e02138.




