
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carmen Freire | + 1392 word(s) | 1392 | 2021-12-28 07:18:39 | | | |
| 2 | Lindsay Dong | Meta information modification | 1392 | 2022-01-05 03:51:27 | | |
Video Upload Options
Natural polymers have emerged as promising candidates for the sustainable development of materials in areas ranging from food packaging and biomedicine to energy storage and electronics.
1. Introduction
2. Applications of Natural Polymers-Based Materials
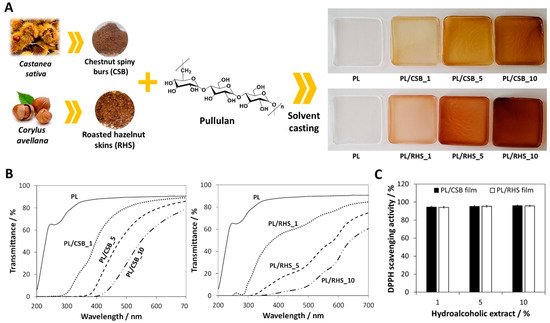
2.1. Natural Polymers-Based Films for Active Food Packaging
2.2. Natural Polymers-Based Ion Exchange Membranes for Fuel Cells
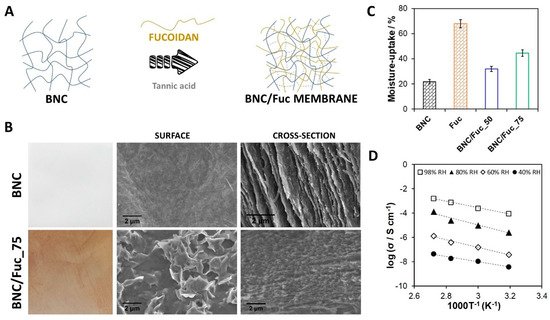
Nanoscale forms of cellulose have garnered great attention for the design of polymer electrolyte fuel cell (PEFC) components, as recently reviewed by Vilela et al. [39]. In particular, BNC-based materials have been studied for the development of sustainable substitutes of ion-exchange membranes. The lack of intrinsic ionic conductivity of BNC can be surpassed with the introduction of ion-conducting phases in the cellulose nanofibrillar structure [39]. In this sense, BNC has been combined with synthetic polyelectrolytes, such as NafionTM [40], poly(4-styrene sulfonic acid) [41][42][43], phosphate bearing monomers [44][45], and poly(ionic liquids) [46], to create partially biobased separators. Particularly relevant is the partnership between BNC and natural polyelectrolytes [47][48] for the design of mechanically and thermally robust fully biobased polymer electrolyte membranes (PEMs). Fucoidan and BNC membranes [47] are examples of these materials, prepared via the simple diffusion of aqueous fucoidan solutions into the exopolysaccharide network and subsequent thermal cross-linking with a natural agent, viz. tannic acid (Figure 2A). Micrographs of the surface and cross-section of the membranes disclosed the well-dispersed sulfate moieties of the algal polysaccharide in the BNC network (Figure 2B). The nanocomposite revealed good dynamic mechanical performance (storage modulus ≥ 460 MPa) and thermal-oxidative stability (180–200 °C) in both inert and oxidative atmospheres. Moreover, this fully biobased ion-exchange membrane displayed good moisture-uptake capacity (Figure 2C) and protonic conductivity, with a maximum of 1.6 mS cm−1 measured at 94 °C and 98% relative humidity conditions (Figure 2D). In general, fully biobased membranes display lower ionic conductivity (when compared with partially biobased counterparts) associated with a smaller content of moieties that enable ion motion. Nevertheless, the fabrication methodologies are simpler, and the resulting materials are entirely environmentally friendly.
2.3. Natural Polymers-Based Patches for Drug Delivery and Wound Healing
Works of BNC [49] and CNFs-based patches [50] have been described as alternatives to non-biodegradable synthetic materials for cutaneous wound healing applications. Aside from the physical protection granted by these systems, the incorporation of antimicrobial agents (e.g., LNFs [50]) or active pharmaceutical ingredients that stimulate cell proliferation (e.g., dexpanthenol [49]) within the patches prevents skin infections and promotes wound closure. In terms of drug-delivery options, nanocellulose patches offer the possibility of self-administering therapeutic molecules using oral, buccal, sublingual, and transdermal routes, which can significantly improve drug bioavailability and minimize side effects by avoiding the first-pass effect on metabolically active tissues of the body [51].
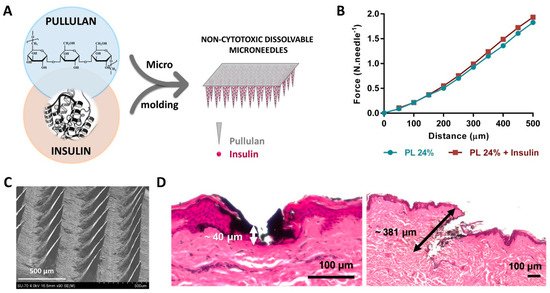
2.4. Natural Polymers-Based Microneedles for Drug Delivery and Fluid Uptake
2.5. Natural Polymers-Based Materials for Other Applications
References
- Yu, L.; Chen, L. Polymeric materials from renewable resources. In Biodegradable Polymer Blends and Composites from Renewable Resources; Yu, L., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 1–15. ISBN 9780470146835.
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393.
- El Knidri, H.; Laajeb, A.; Lahsini, A. Chitin and chitosan: Chemistry, solubility, fiber formation, and their potential applications. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 35–57. ISBN 978-0-12-817970-3.
- Silvetti, T.; Morandi, S.; Hintersteiner, M.; Brasca, M. Use of hen egg white lysozyme in the food industry. In Egg Innovations and Strategies for Improvements; Hester, P.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 233–242. ISBN 9780128011515.
- Vilela, C.; Pinto, R.J.B.; Pinto, S.; Marques, P.A.A.P.; Silvestre, A.J.D.; Freire, C.S.R. Polysaccharide Based Hybrid Materials: Metals and Metal Oxides, Graphene and Carbon Nanotubes, 1st ed.; Springer Briefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2018; ISBN 9783030003463.
- Silva, N.H.C.S.; Vilela, C.; Marrucho, I.M.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Protein-Based materials: From sources to innovative sustainable materials for biomedical applications. J. Mater. Chem. B 2014, 2, 3715–3740.
- Basu, S.; Malik, S.; Joshi, G.; Gupta, P.; Rana, V. Utilization of bio-polymeric additives for a sustainable production strategy in pulp and paper manufacturing: A comprehensive review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100050.
- Silva, A.C.Q.; Silvestre, A.J.D.; Freire, C.S.R.; Vilela, C. Modification of textiles for functional applications. In Fundamentals of Natural Fibres and Textiles; Mondal, M.I.H., Ed.; Woodhead Publishing, Elsevier Ltd.: Amsterdam, The Netherlands, 2021; pp. 303–365. ISBN 9780128214848.
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-Induced gelation of alginate: Mechanisms and applications. Int. J. Biol. Macromol. 2021, 177, 578–588.
- Mostafavi, F.S.; Zaeim, D. Agar-Based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176.
- Alipal, J.; Pu’Ad, N.M.; Lee, T.; Nayan, N.; Sahari, N.; Basri, H.; Idris, M.; Abdullah, H. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today Proc. 2021, 42, 240–250.
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods. Mar. Drugs 2018, 16, 321.
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695.
- Gradishar, W.J.; Tjulandin, S.; Davidson, N.; Shaw, H.; Desai, N.; Bhar, P.; Hawkins, M.; O’Shaughnessy, J. Phase III Trial of Nanoparticle Albumin-Bound Paclitaxel Compared with Polyethylated Castor Oil–Based Paclitaxel in Women with Breast Cancer. J. Clin. Oncol. 2005, 23, 7794–7803.
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501.
- Ibrahim, S.; Riahi, O.; Said, S.M.; Sabri, M.F.M.; Rozali, S. Biopolymers From Crop Plants. In Reference Module in Materials Science and Materials Engineering; Elsevier: Hoboken, NJ, USA, 2019; pp. 1–10.
- Tongdeesoontorn, W.; Rawdkuen, S. Gelatin-Based Films and Coatings for Food Packaging Applications. In Reference Module in Food Science; Elsevier: Hoboken, NJ, USA, 2019; pp. 1–15.
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346.
- Mansoori, S.; Davarnejad, R.; Matsuura, T.; Ismail, A.F. Membranes based on non-synthetic (natural) polymers for wastewater treatment. Polym. Test. 2020, 84, 106381.
- Vedula, S.S.; Yadav, G.D. Chitosan-based membranes preparation and applications: Challenges and opportunities. J. Indian Chem. Soc. 2021, 98, 100017.
- Nechita, P.; Roman, M. Review on Polysaccharides Used in Coatings for Food Packaging Papers. Coatings 2020, 10, 566.
- Song, J.; Winkeljann, B.; Lieleg, O. Biopolymer-Based Coatings: Promising Strategies to Improve the Biocompatibility and Functionality of Materials Used in Biomedical Engineering. Adv. Mater. Interfaces 2020, 7, 2000850.
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364.
- Carvalho, J.P.F.; Silva, A.C.Q.; Silvestre, A.J.D.; Freire, C.S.R.; Vilela, C. Spherical Cellulose Micro and Nanoparticles: A Review of Recent Developments and Applications. Nanomaterials 2021, 11, 2744.
- Bennacef, C.; Desobry-Banon, S.; Probst, L.; Desobry, S. Advances on alginate use for spherification to encapsulate biomolecules. Food Hydrocoll. 2021, 118, 106782.
- DeFrates, K.; Markiewicz, T.; Gallo, P.; Rack, A.; Weyhmiller, A.; Jarmusik, B.; Hu, X. Protein Polymer-Based Nanoparticles: Fabrication and Medical Applications. Int. J. Mol. Sci. 2018, 19, 1717.
- Vilela, C.; Moreirinha, C.; Domingues, E.M.; Figueiredo, F.M.L.; Almeida, A.; Freire, C.S.R. Antimicrobial and Conductive Nanocellulose-Based Films for Active and Intelligent Food Packaging. Nanomaterials 2019, 9, 980.
- Bastante, C.C.; Silva, N.H.; Cardoso, L.C.; Serrano, C.M.; de la Ossa, E.J.M.; Freire, C.S.; Vilela, C. Biobased films of nanocellulose and mango leaf extract for active food packaging: Supercritical impregnation versus solvent casting. Food Hydrocoll. 2021, 117, 106709.
- Moreirinha, C.; Vilela, C.; Silva, N.H.; Pinto, R.J.; Almeida, A.; Rocha, M.A.M.; Coelho, E.; Coimbra, M.A.; Silvestre, A.J.; Freire, C.S. Antioxidant and antimicrobial films based on brewers spent grain arabinoxylans, nanocellulose and feruloylated compounds for active packaging. Food Hydrocoll. 2020, 108, 105836.
- Tomé, L.C.; Silva, N.H.C.S.; Soares, H.R.; Coroadinha, A.S.; Sadocco, P.; Marrucho, I.M.; Freire, C.S.R. Bioactive transparent films based on polysaccharides and cholinium carboxylate ionic liquids. Green Chem. 2015, 17, 4291–4299.
- Esposito, T.; Silva, N.H.; Almeida, A.; Silvestre, A.J.; Piccinelli, A.L.; Aquino, R.P.; Sansone, F.; Mencherini, T.; Vilela, C.; Freire, C.S. Valorisation of chestnut spiny burs and roasted hazelnut skins extracts as bioactive additives for packaging films. Ind. Crops Prod. 2020, 151, 112491.
- Pinto, R.; Almeida, A.; Fernandes, S.C.; Freire, C.; Silvestre, A.; Neto, C.P.; Trindade, T. Antifungal activity of transparent nanocomposite thin films of pullulan and silver against Aspergillus niger. Colloids Surf. B Biointerfaces 2013, 103, 143–148.
- Vilela, C.; Pinto, R.J.; Coelho, J.; Domingues, M.R.; Daina, S.; Sadocco, P.; Santos, S.A.; Freire, C. Bioactive chitosan/ellagic acid films with UV-light protection for active food packaging. Food Hydrocoll. 2017, 73, 120–128.
- Makhloufi, N.; Chougui, N.; Rezgui, F.; Benramdane, E.; Freire, C.S.R.; Vilela, C.; Silvestre, A.J.D. Bio-based sustainable films from the Algerian Opuntia ficus-indica cladodes powder: Effect of plasticizer content. J. Appl. Polym. Sci. 2021, 138, 50450.
- Carvalho, J.P.F.; Freire, C.S.R.; Vilela, C. Active Packaging. In Sustainable Food Processing and Engineering Challenges; Galanakis, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 315–341. ISBN 9780128227145.
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222.
- Silva, N.H.; Vilela, C.; Almeida, A.; Marrucho, I.; Freire, C. Pullulan-based nanocomposite films for functional food packaging: Exploiting lysozyme nanofibers as antibacterial and antioxidant reinforcing additives. Food Hydrocoll. 2018, 77, 921–930.
- Silva, N.H.; Vilela, C.; Pinto, R.J.; Martins, M.A.; Marrucho, I.M.; Freire, C.S. Tuning lysozyme nanofibers dimensions using deep eutectic solvents for improved reinforcement ability. Int. J. Biol. Macromol. 2018, 115, 518–527.
- Vilela, C.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Nanocellulose-based materials as components of polymer electrolyte fuel cells. J. Mater. Chem. A 2019, 7, 20045–20074.
- Gadim, T.D.; Vilela, C.; Loureiro, F.; Silvestre, A.; Freire, C.S.; Figueiredo, F.M. Nafion ® and nanocellulose: A partnership for greener polymer electrolyte membranes. Ind. Crops Prod. 2016, 93, 212–218.
- Gadim, T.D.O.; Figueiredo, A.G.P.R.; Navarro, N.C.R.; Vilela, C.; Gamelas, J.; Timmons, A.B.; Neto, C.P.; Silvestre, A.; Freire, C.; Figueiredo, F.M.L. Nanostructured Bacterial Cellulose–Poly(4-styrene sulfonic acid) Composite Membranes with High Storage Modulus and Protonic Conductivity. ACS Appl. Mater. Interfaces 2014, 6, 7864–7875.
- Gadim, T.D.; Loureiro, F.J.; Vilela, C.; Rosero-Navarro, N.; Silvestre, A.J.; Freire, C.S.; Figueiredo, F.M. Protonic conductivity and fuel cell tests of nanocomposite membranes based on bacterial cellulose. Electrochim. Acta 2017, 233, 52–61.
- Vilela, C.; Cordeiro, D.M.; Boas, J.V.; Barbosa, P.; Nolasco, M.; Vaz, P.D.; Rudić, S.; Ribeiro-Claro, P.; Silvestre, A.J.; Oliveira, V.B.; et al. Poly(4-styrene sulfonic acid)/bacterial cellulose membranes: Electrochemical performance in a single-chamber microbial fuel cell. Bioresour. Technol. Rep. 2019, 9, 100376.
- Vilela, C.; Martins, A.P.C.; Sousa, N.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Poly(bis phosphate)/Bacterial Cellulose Nanocomposites: Preparation, Characterization and Application as Polymer Electrolyte Membranes. Appl. Sci. 2018, 8, 1145.
- Vilela, C.; Gadim, T.D.O.; Silvestre, A.; Freire, C.; Figueiredo, F.M.L. Nanocellulose/poly(methacryloyloxyethyl phosphate) composites as proton separator materials. Cellulose 2016, 23, 3677–3689.
- Vilela, C.; Sousa, N.; Pinto, R.; Silvestre, A.; Figueiredo, F.M.; Freire, C.S. Exploiting poly(ionic liquids) and nanocellulose for the development of bio-based anion-exchange membranes. Biomass Bioenergy 2017, 100, 116–125.
- Vilela, C.; Silva, A.C.; Domingues, E.; Gonçalves, G.; Martins, M.A.; Figueiredo, F.M.; Santos, S.A.; Freire, C.S. Conductive polysaccharides-based proton-exchange membranes for fuel cell applications: The case of bacterial cellulose and fucoidan. Carbohydr. Polym. 2020, 230, 115604.
- Vilela, C.; Morais, J.D.; Silva, A.C.Q.; Muñoz-Gil, D.; Figueiredo, F.M.L.; Silvestre, A.J.D.; Freire, C.S.R. Flexible Nanocellulose/Lignosulfonates Ion-Conducting Separators for Polymer Electrolyte Fuel Cells. Nanomaterials 2020, 10, 1713.
- Fonseca, D.F.S.; Carvalho, J.P.F.; Bastos, V.; Oliveira, H.; Moreirinha, C.; Almeida, A.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Antibacterial Multi-Layered Nanocellulose-Based Patches Loaded with Dexpanthenol for Wound Healing Applications. Nanomaterials 2020, 10, 2469.
- Silva, N.H.; Garrido-Pascual, P.; Moreirinha, C.; Almeida, A.; Palomares, T.; Alonso-Varona, A.; Vilela, C.; Freire, C.S. Multifunctional nanofibrous patches composed of nanocellulose and lysozyme nanofibers for cutaneous wound healing. Int. J. Biol. Macromol. 2020, 165, 1198–1210.
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574.
- Fonseca, D.; Vilela, C.; Silvestre, A.J.; Freire, C.S. A compendium of current developments on polysaccharide and protein-based microneedles. Int. J. Biol. Macromol. 2019, 136, 704–728.
- Fonseca, D.F.; Costa, P.C.; Almeida, I.F.; Dias-Pereira, P.; Correia-Sá, I.; Bastos, V.; Oliveira, H.; Duarte-Araújo, M.; Morato, M.; Vilela, C.; et al. Pullulan microneedle patches for the efficient transdermal administration of insulin envisioning diabetes treatment. Carbohydr. Polym. 2020, 241, 116314.
- Fonseca, D.F.; Vilela, C.; Pinto, R.J.; Bastos, V.; Oliveira, H.; Catarino, J.; Faísca, P.; Rosado, C.; Silvestre, A.J.; Freire, C.S. Bacterial nanocellulose-hyaluronic acid microneedle patches for skin applications: In vitro and in vivo evaluation. Mater. Sci. Eng. C 2021, 118, 111350.
- Fonseca, D.F.S.; Costa, P.C.; Almeida, I.F.; Dias-Pereira, P.; Correia-Sá, I.; Bastos, V.; Oliveira, H.; Vilela, C.; Silvestre, A.J.D.; Freire, C.S.R. Swellable Gelatin Methacryloyl Microneedles for Extraction of Interstitial Skin Fluid toward Minimally Invasive Monitoring of Urea. Macromol. Biosci. 2020, 20, e2000195.
- Carneiro, J.; Tedim, J.; Fernandes, S.; Freire, C.; Gandini, A.; Ferreira, M.; Zheludkevich, M. Functionalized chitosan-based coatings for active corrosion protection. Surf. Coat. Technol. 2013, 226, 51–59.
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.; Gandini, A.; Ferreira, M.; Zheludkevich, M. Chitosan as a Smart Coating for Controlled Release of Corrosion Inhibitor 2-Mercaptobenzothiazole. ECS Electrochem. Lett. 2013, 2, C19–C22.
- Carneiro, J.; Tedim, J.; Fernandes, S.; Freire, C.; Silvestre, A.; Gandini, A.; Ferreira, M.; Zheludkevich, M. Chitosan-based self-healing protective coatings doped with cerium nitrate for corrosion protection of aluminum alloy 2024. Prog. Org. Coat. 2012, 75, 8–13.
- Pinto, R.J.B.; Martins, M.A.; Lucas, J.M.F.; Vilela, C.; Sales, A.; Costa, L.C.; Marques, P.; Freire, C.S.R. Highly Electroconductive Nanopapers Based on Nanocellulose and Copper Nanowires: A New Generation of Flexible and Sustainable Electrical Materials. ACS Appl. Mater. Interfaces 2020, 12, 34208–34216.
- Inácio, P.M.; Medeiros, M.D.C.; Carvalho, T.; Félix, R.C.; Mestre, A.; Hubbard, P.C.; Ferreira, Q.; Morgado, J.; Charas, A.; Freire, C.S.; et al. Ultra-low noise PEDOT:PSS electrodes on bacterial cellulose: A sensor to access bioelectrical signals in non-electrogenic cells. Org. Electron. 2020, 85, 105882.
- Pinto, R.J.B.; Granadeiro, C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Ferreira, R.A.S.; Carlos, L.D.; Cavaleiro, A.M.V.; Trindade, T.; Nogueira, H.I.S. Luminescent Transparent Composite Films Based on Lanthanopolyoxometalates and Filmogenic Polysaccharides. Eur. J. Inorg. Chem. 2013, 2013, 1890–1896.
- Barata, J.F.B.; Pinto, R.J.B.; Serra, V.I.R.C.V.; Silvestre, A.J.D.; Trindade, T.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Daina, S.; Sadocco, P.; Freire, C.S.R. Fluorescent Bioactive Corrole Grafted-Chitosan Films. Biomacromolecules 2016, 17, 1395–1403.
- Pinto, R.J.B.; Lameirinhas, N.S.; Guedes, G.; da Silva, G.H.R.; Oskoei, P.; Spirk, S.; Oliveira, H.; Duarte, I.F.; Vilela, C.; Freire, C.S.R. Cellulose Nanocrystals/Chitosan-Based Nanosystems: Synthesis, Characterization, and Cellular Uptake on Breast Cancer Cells. Nanomaterials 2021, 11, 2057.
- Vilela, C.; Moreirinha, C.; Almeida, A.; Silvestre, A.J.D.; Freire, C.S.R. Zwitterionic Nanocellulose-Based Membranes for Organic Dye Removal. Materials 2019, 12, 1404.
- Silva, N.H.; Figueira, P.; Fabre, E.; Pinto, R.J.; Pereira, M.E.; Silvestre, A.J.; Marrucho, I.M.; Vilela, C.; Freire, C.S. Dual nanofibrillar-based bio-sorbent films composed of nanocellulose and lysozyme nanofibrils for mercury removal from spring waters. Carbohydr. Polym. 2020, 238, 116210.




