1. Background
CO2 reutilization processes contribute to the mitigation of CO2 as a potent greenhouse gas (GHG) through reusing and converting it into economically valuable chemical products including methanol, dimethyl ether, and methane. Solar thermochemical conversion and photochemical and electrochemical CO2 reduction processes are emerging technologies in which solar energy is utilized to provide the energy required for the endothermic dissociation of CO2. Owing to the surface-dependent nature of these technologies, their performance is significantly reliant on the solid reactant/catalyst accessible surface area. Solid porous structures either entirely made from the catalyst or used as a support for coating the catalyst/solid reactants can increase the number of active reaction sites and, thus, the kinetics of CO2 reutilization reactions.
2. Application of Porous Materials in Solar Thermochemical Conversion
Re-oxidation of the reduced RedOx materials during the thermochemical cycles is strongly dependent on the available solid surface area for the reactions. Thus, the use of porous materials, enabling a high surface-to-volume (S/V) ratio, is substantially advantageous in terms of reaction kinetics and mass transfer
[1][2][3][4][5][6][7][8][9][10][11][12][13][14]. Different types of porous materials including reticulated porous ceramics (RPCs)
[4][5][8][15], honeycombs
[16][17], foams
[1][6][12][13][18], and felts as well as 3D ordered macroporous materials have been utilized successfully in this regard. These materials were either applied entirely made of active RedOx agent or as a thin layer of RedOx coating over a stable structure. The pore size of the RPCs can be classified as micro (<2 nm), meso (2–50 nm), and macro (>50 nm)
[19]. In the following sections, the application of porous materials in solar thermochemical CO
2 conversion is reviewed. In addition, a chronical list of works on solar thermochemical conversion processes are presented in
Table 1. Since the oxide materials for carbon dioxide and water splitting can be used interchangeably in a similar series of reactions (Equations (2) and (3)), the application of porous materials in both CO
2 and water splitting are summarized here.
Table 1. List of research items on the application of porous materials for solar thermochemical conversion processes.
| Year |
Porous Support |
RedOx Coating Material |
Chemical Process |
Performance |
References |
| 1989 |
Alumina honeycomb/foam |
Rh |
CO2 methane reforming |
more efficiency for honeycomb structure compared to foams |
[145,146] |
| 2005 |
re-crystallized SiC honeycomb |
Mn/Zn ferrites |
water splitting |
conversion efficiency ~80% and hydrogen yield >90% at low oxidation temperatures (800 °C) |
[141,147] |
| 2008 |
c-YSZ / MPSZ foam |
Fe3O4 |
water splitting |
ferrite conversion of 20–27% for a 10.5 wt% Fe3O4-coated porous MPSZ |
[137] |
| 2009 |
MPSZ foam |
m-ZrO2 supported NiFe2O4, Fe3O4 |
water splitting |
ferrite conversion of 24–76% for a 25 wt%
NiFe2O4 coating on porous MPSZ |
[97] |
| 2008 1 |
1:3 Co0.67Fe2.33O4/YSZ, Al2O3 and TiO2 |
- |
water splitting |
unfavorable side reactions of ferrite with the YSZ supports and, thus, weak performance of the porous RedOx material |
[127,148] |
| 2010 |
cerium oxide (CeO2) monolith |
- |
water splitting/carbon dioxide splitting |
with ƞsolar-to-fuel = 0.7–0.8% and the possibility of improvement through upscaling and removing the heat losses’ effects |
[70] |
| 2011 |
siliconized SiC monoliths |
Fe/Zn mixed oxide |
water splitting |
conversion efficiency ~30% and RedOx materials’ degradation due to zinc content volatilization and inhomogeneous temperature distribution |
[149] |
| MPSZ foam |
zirconia supported Fe3O4 or NiFe2O4 |
water splitting |
maximum ferrite conversion of 60% for NiFe2O4/m-ZrO2/MPSZ foam device |
[143] |
| 2012 2 |
75 vol% YSZ and 25 vol% Fe2O3 |
- |
carbon dioxide splitting |
yttria addition led to the oxygen conductivity improvement and Iron oxide conversion (max: 58%) and the stability of the CO production in consecutive RedOx cycles. The increasing of co-extruded honeycomb substrates’ surface area from ~2.6 to ~8.5 cm2 did not lead to a notable improvement in CO generation per unit volume |
[142] |
| porous ceria felt |
- |
water splitting/carbon dioxide splitting |
ceria sublimation and deposition on reactor components were detected as the main technical challenges, which eventually were responsible for deterioration of the active material and, thus, reactor yield |
[150] |
| ceria RPC |
- |
carbon dioxide splitting |
with mean ƞsolar-to-fuel = 1.73% and max ƞsolar-to-fuel = 3.53%,
a 17 times’ improvement in the fuel yield per cycle compared to ceria felt in previous study |
[114] |
| 2013 |
3DOM 3 CeO2, NOM 4 CeO2 |
- |
carbon dioxide splitting |
more structural stability and CO production rate (10-fold) of porous structures over non-porous ones in over 55 cycles |
[151] |
| 2014 |
ceria RPC with dual porosities |
- |
carbon dioxide splitting |
a 10 times higher yield for samples with porous struts (44% porosity) compared to samples with non-porous solid struts. The mean ηsolar-to-fuel of 1.72% was also detected in a 3.8-kW solar cavity receiver |
[118] |
| MPSZ |
NiFe2O4/m-ZrO2 and CeO2 |
water splitting |
lower yields of NiFe2O4/m-ZrO2/MPSZ compared to CeO2/MPSZ as a result of some sintering effects |
[138] |
| SiC, Ni, Cu foams |
ZrO2-supported CeO2 |
methane reforming/water splitting |
higher gas yields for the Ni and Cu foams than for SiC. The poor thermal conductivity of SiC foam was also responsible for CeO2 particle sintering and, thus, an overall efficiency decrement. |
[112] |
| 2015 |
porous ceria |
- |
carbon dioxide splitting |
high degree of reactivity (even after 2000 cycles) was reported |
[152] |
| 2017 |
ceria RPC with dual porosities |
- |
carbon dioxide splitting |
molar CO2 conversion of 83% and ƞsolar-to-fuel = 5.25% |
[139,153] |
| 2019 |
Ceria RPC |
- |
water splitting/carbon dioxide splitting |
max. ƞsolar-to-fuel = 5.22% while increasing methane flow rate and decreasing the reduction temperature will enhance the nonstoichiometry value and, thus, syngas yield |
[154] |
| 2020 |
SiC RPC with dual porosities |
La0.6Ca0.4Mn0..6Al0.4O3±δ (LCMA) |
carbon dioxide splitting |
CO2 conversion with [CO%] = 3.2. A coagulation of smaller pores because of reaction between LCMA coating and SiC substrate was reported. The smallest pore size of 75 ppi delivered the highest CO yield of ca. 0.07 molg−1 LCMA and δ = 0.4 |
[18,136] |
| ceria RPC (with gradient porosities 10–60 ppi) |
- |
water splitting/carbon dioxide splitting |
max. ηsolar-to-fuel of ~7.5% after 64 cycles was measured with a high stability of the porous RedOx structures |
[73] |
| ceria RPC |
La0.5Sr0.5Mn0.9Mg0.1O3 (LSMMg) |
water splitting/carbon dioxide splitting |
max. ηsolar-to-fuel = 5.3 and the perovskite coating had just a positive effect on the reduction extent, which hindered oxidant gas (H2O or CO2) accessing the reactive ceria and can result in the poor re-oxidation compared to the pure, uncoated ceria RPC |
[14] |
The very first application of porous materials in solar thermochemical CO2 utilization dates back to 1989 when the deposition of Rh on an alumina-based honeycomb or foam structures for CO2 methane reforming was investigated
[20][21]. In this study, the performance of the honeycomb structure (4-mm square holes, 0.5-mm wall thickness) proved to be more efficient than that of the 10-ppi (pores per inch) foam. Nevertheless, it was conducted to make a comprehensive conclusion, and more assessment is needed. In the HYDROSOL project in 2005, a monolithic honeycomb medium made from re-crystallized silicon carbide (reSiC) was used in the framework of a solar reactor (
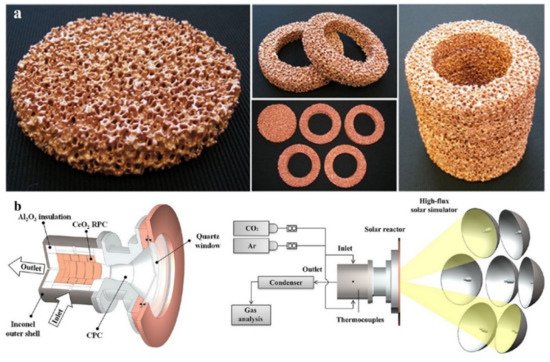
Figure 1)
[16]. The porous materials were structurally similar to the automobile catalyst media and consisted of Mn/Zn ferrites coated on reSiC honeycombs. They were tested within a series of reduction (1300 °C) and oxidation (800 °C) reactions for water splitting. The reactor reached a conversion efficiency of ~ 80% and hydrogen yield of >90% at oxidation temperatures as low as 800 °C. The measured hydrogen evolved in these experiments was in a good agreement with the laboratory experiments with the oxides in the form of powders, showing that the RedOx materials coated on a porous substrate with mean d
p = 6 µm maintained their reactivity
[16][22]. The main advantage of the HYDROSOL reactor is that it has no moving parts or even particles. Moreover, it enables controlling the temperature and the reactor operation through adjusting the mass flux density in each reactor module, in response to the variations in the inlet solar heat flux. This is significantly important given the diurnal and annual variations of the solar insolation.
Figure 1. The HYDROSOL solar receiver-reactor used for water splitting showing the installment position and configuration of Mn/Zn-ferrite coated SiC honeycombs
[16].
The next work in this field was published in 2008 when Gokon et al.
[12] conducted an experimental study on the application of Fe
3O
4, as RedOx agent, coated on both cubic yttria-stabilized zirconia (Fe
3O
4/c-YSZ) and magnesia partially stabilized zirconia (MPSZ) to compare the potentials for water splitting and production of hydrogen. The aerial oxidation of the aqueous suspensions of Fe(II) hydroxide was used to coat Fe
3O
4 on zirconia doped with 8 mol% Y
2O
3 as the active powder material. To coat MPSZ substrates, YSZ was loaded on the porous MPSZ to increase the surface area. The structure was then impregnated into the iron nitrate solution to be coated with Fe
3O
4. Coating of Fe
3O
4 on a substrate mainly prevents the coagulation or sintering of particles. The thermal reduction and water splitting reactions were performed in two different reactors equipped with infrared furnaces. The results revealed a ferrite conversion of 20–27% for a 10.5 wt.% Fe
3O
4-coated porous MPSZ after 32 consecutive cycles of hydrogen production (TR: 1400–1450 °C and WS: 1100 °C) and an irradiation period of 60 min during each RedOx cycle. However, it seems that performing thermochemical reactions in two separate reactors could lead to some uncertainty in the measurements, while the use of a single reactor for both RedOx reactions may enable a better control over the reactor atmosphere and the inlet/outlet gases and, hence, more accurate measurements. In their next work
[6], monoclinic zirconia (m-ZrO
2)-supported NiFe
2O
4 and Fe
3O
4 powders were similarly tested in powder and coated on MPSZ foam for water splitting process. A ferrite conversion of 24–76% was obtained after 10 repeated cycles and an irradiation period of 30 min in each cycle. These demonstrated the competency of foam-like RedOx materials compared to powder materials and the significance of the surface area in reactions.
Due to the significance of the surface-to-volume (S/V) ratio, a substantial attempt was also allocated to the direct fabrication of the RedOx powders into monolithic structures through methods like robocasting and slurry processes
[23][24]. For example, cast 3D lattice-structured monoliths were manufactured from 1: 3Co
0.67Fe
2.33O
4: YSZ to enhance the vacancies within the structure and, thus, oxygen mass transfer within solids. Similarly, polymethylmethacrylate (PMMA) as pore former was added to the materials to increase porosity within rods. These porous networks were utilized in cyclic lab scale and on sun water splitting reactions within the temperature range of 1000–1400 °C. Although the structure’s integrity was improved, the 1:3 Co
0.67Fe
2.33O
4:YSZ showed limited capability for the cyclic water splitting, due to unfavorable side reactions of ferrite with the YSZ supports
[23].
Porous, monolithic ceria was used in a directly irradiated solar cavity receiver/reactor for H
2O and CO
2 splitting. The material demonstrated stable and rapid oxidation reactions for CO and H
2 production over 500 cycles with ƞ
solar-to-fuel = 0.7–0.8%, which was argued to be dependent on the system scale and design rather than materials’ chemistry. That is because both the reaction rates and the efficiency of the reactor were found to be limited mainly by the thermal losses as a result of both convective and radiative heat losses
[25].
In another work, two adjacent thermochemical reactors consisting of nine siliconized silicon carbide (SiSiC) monoliths (146 × 146 mm
2) coated with iron–zinc mixed oxide were employed as chambers within a 100-kW
th directly irradiated solar water splitting reactor with a reduction temperature of ~1200 °C, followed by water splitting at 800 °C
[26]. The conversion of steam was reported to be ~ 30%, while slight degradation and deactivation of the RedOx material were also observed, which were due to both the volatilization of the zinc oxide content of the porous structure and inhomogeneous temperature distribution within the solar reactor. Nonetheless, this was a successful demonstration of the use of RedOx-coated porous absorbers at a pilot-scale solar receiver/reactor. Gokon et al.
[18] utilized ferrite/zirconia foam spin-coated with zirconia-supported Fe
3O
4 or NiFe
2O
4. i.e., Fe
3O
4/m-ZrO
2, Fe
3O
4/c-YSZ, NiFe
2O
4/m-ZrO
2, and NiFe
2O
4/c-YSZ coated on MPSZ as RedOx materials. These materials were tested for the two-step water splitting in a directly irradiated solar reactor by means of a Xe lamp solar simulator with a power output of 4 × 10
6 kW
th. The highest reactivity was observed from the NiFe
2O
4/m-ZrO
2 pair coated on MPSZ with a relatively constant hydrogen production rate and maximum ferrite conversion of 60% in 20 consecutive cycles
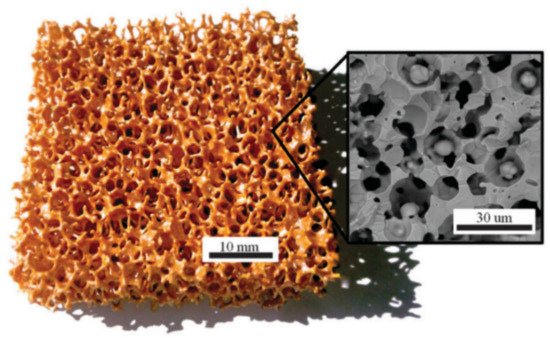
[17]. Polymer-based coextrusion ceramic honeycombs of zirconia and iron oxide (
Figure 2) were used for splitting of carbon dioxide. This synthesis method enables controlling the surface area of the honeycombs. Moreover, the addition of 3 mol.% and 8 mol.% of yttria improved the oxygen conductivity of the materials, leading to a noticeable increase in both iron oxide conversion from 41% to 58% and the stability of the CO production in consecutive RedOx cycles. Additionally, the results showed that the CO generation significantly depended on the reaction temperature and CO
2 flow rate. Interestingly, the increasing of substrates’ surface area from ~2.6 to ~8.5 cm
2 did not lead to a significant improvement in CO generation per unit volume of the extruded honeycomb structure, as it did not significantly differ in terms of pore size. The reaction mechanism was also found to be initially spontaneous over the surface of the RedOx materials, while, over time, the diffusion mass transfer through pore structure became dominant and the reaction rate controlling, which can be attributed to the enhanced transport phenomena
[17].
Figure 2. Polymer-based coextrusion ceramic honeycombs made from homogeneous composites of iron oxide and zirconia for the solar thermochemical dissociation of CO
2 to CO
[17].
Furler et al.
[27] examined porous ceria felt for thermochemical CO
2 and water splitting in a solar directly irradiated cavity-receiver, exposed to a mean solar concentration ratio of 2865 suns (TR: 1527 °C and WS/CDS: 827 °C). The dependence of the composition of the product syngas on the composition of the feeding mixture of CO
2 and H
2O in a range of 0.8 to 7.7 was investigated. Advantageously, it has been found that the composition of the product syngas can be optimized enabling a H
2 to CO ratio of ~2, which is needed in Fischer-Tropsch processes, through varying the feeding ratio of the H
2O to CO
2 for the oxidation of the porous ceria felt. Nevertheless, ceria sublimation and deposition on other reactor components, in particular irradiation widow, were found as the main technical challenges, which can lead to thermal stress and crack in the reactor structure. Sublimation of the ceria also leads to the loss of active materials and reduction of the oxygen transfer capacity and hence reactor yield over consecutive RedOx cycles. This directly irradiated solar reactor was further scaled up to accommodate four RPC rings, entirely made from ceria as depicted in
Figure 3a
[8]. A mean solar to fuel energy conversion efficiency of 1.73% together with a maximum spontaneous efficiency of 3.53% was reported for CO
2 splitting. They also observed oxygen deficiency (δ), ranging from 0.016 to 0.04 for the temperatures of 1400 to 1600 °C. Also, the use of porous RedOx material in this setup (
Figure 3b) was compared with the optically thick ceria felt used in their previous study
[27] and confirmed a 17 times improvement in the fuel yield per cycle.
Figure 3. (
a) Ceria-coated RPCs consisting of a 20-mm thickness, 100-mm o.d. disk, and four 20-mm thicknesses, 60-mm i.d., 100-mm o.d. rings; (
b) High-Flux Solar Simulator and the configuration of porous modules’ assembly at the Swiss Federal Institute of Technology (ETH) (bottom)
[8].
Three-dimensionally macro-porous (3DOM) CeO
2 and non-ordered macroporous (NOM) CeO
2 were also used for CO
2 splitting
[28]. These structures were synthesized using polymeric colloidal spheres as templates and tested in an infrared furnace (TR: 1200 , CDS: 850). The results in this case confirmed the advantages of using porous over non-porous structures, as the structure of the porous RedOx materials was almost stable in over 55 cycles, leading to a considerable improvement in the CO production rate (10-fold enhancement) relative to that of the nonporous CeO
2 structure. This was attributed to the differences in reactivity of materials because of differences in their surface accessibility by the oxidative gas through the oxidation cycle. This research revealed the importance of pore engineering of porous materials through pore templating that could potentially improve the interconnectivity of the three-dimensional pore structure and mass transfer, especially through the oxidation stage, and, thus, the kinetics of the oxidation step besides improving the resistivity toward sintering.
The novel configuration of ceria RPCs with dual scale porosity, i.e., larger macropores, between struts and smaller µm-sized pores inside struts, was also examined
[9]. In doing so, some ceria foams were manufactured with pore sizes in the millimeter range (d
mean = 2.5 mm and ε = 0.76–0.82) macroscopically through the bulk and micrometer order (d
mean = 10 μm and ε
strut = 0–0.44) inside the struts by using some sacrificial carbon-based pore-forming agent with particle sizes of 0.4–12 µm. The synthesized dual-scale ceria foam is shown in
Figure 4. Thermogravimetric experiments (TR: 1500 °C and CDS: 600–1000 °C) revealed a 10 times’ higher yield for the samples with porous struts (porosity: 0.44) compared to samples with non-porous solid struts. It was hypothesized that the millimeter pores could enhance the reduction reaction, providing an improved penetration of thermal irradiation into depth and, thus, a uniform heating, while the micrometer pores could help the oxidation kinetics by providing a better surface area for such a surface-limited process and decreasing the oxidation time to about one-ninth. Indeed, there was trade-off between the specific mass of active RedOx material per unit volume and porosity. While a higher specific mass led to a higher conversion, due to the availability of more active materials, a low porosity resulted in less radiation penetration and, hence, reduction extent. Finally, for a validation of TGA results, the dual-scale RPC was used in a solar cavity-receiver (3.8-kW radiative power at 3015 suns) and showed a mean η
solar-to-fuel of 1.72%
[14]. MPSZ foams coated with ferrite supported on monoclinic zirconia (NiFe
2O
4/ m-ZrO
2/MPSZ) and cerium oxide (CeO
2/MPSZ) were also tested in a directly irradiated receiver/reactor equipped with a Xe solar simulator for water splitting
[13]. Results showed that the NiFe
2O
4/m-ZrO
2/MPSZ RedOx material had lower yields than CeO
2/MPSZ as a result of some sintering effects at reduction temperatures of TR = 1450 and 1550 °C. The effects of the porous substrates’ materials on the methane reforming for syngas production and water splitting was also investigated
[7]. SiC, Ni, and Cu foams coated with zirconia-supported cerium oxide were heated to 900 °C using a solar simulator. Results revealed higher gas yields for the Ni and Cu foams than for the SiC one. This was related to the catalytic nature of Ni and Cu for the methane reforming reactions and the better radial thermal distribution within these metals. Indeed, the poor thermal conductivity of the SiC foam substrate compared to the metallic ones resulted in CeO
2 particle sintering in consecutive cycles and, thus, a decrement on the overall efficiency.
Figure 4. Ceria RPC with dual-scale porosities; macro-porous structure with mm-sized pores that are ideal for reduction and µm-sized pores inside struts (SEM micrograph) responsible for oxidation reaction in the carbon dioxide splitting process
[9].
The cerium oxide as a RedOx material was examined in various works. For example, the powder and porous forms of the cerium (IV) oxide was tested for CO
2 splitting in a thermogravimetric analyzer (TGA) setup (TR: 1450 °C and CDS: 1100 °C)
[29]. The results showed a mean value (after 2000 cycles) of δ = 0.0197 in CeO
2 → CeO
2−δ cycle with a maximum value of δ = 0.0383 at 1450 °C. Higher degrees of non-stoichiometry (δ) led to more oxygen storage/loss and mobility, while also maintaining the crystallographic fluorite structure of ceria, leading to more fuel production yield. The porous samples were reported to slightly lose some of their surface area. However, the porous structure maintained its reactivity even after 2000 cycles, which is a prerequisite if the RedOx cycle based on the cerium oxide is going to be commercialized.
Some efforts on the bench scale as a prerequisite for large-scale demonstration and commercialization of the RedOx materials have been also reported
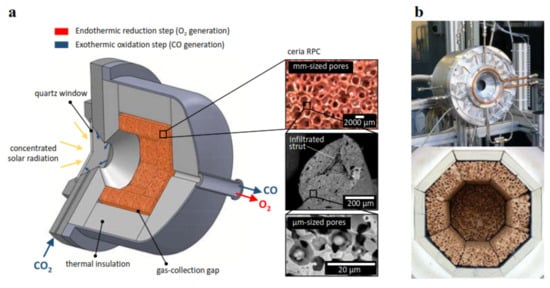
[14][30]. For example, in a recent attempt, a 4-kW solar reactor featuring a ceria RPC with dual porosities as shown in
Figure 5a was developed and utilized for CO
2 splitting
[14]. The thermal reduction was carried out at T
reduction = 1450–1500 °C and vacuum pressures (p
total = 10–1000 mbar) by means of a solar simulator with a power of 2.4–4.1 kW. By turning off the solar simulator and cooling down into T
oxidation = 700–1000 °C, the oxidation reaction occurred at CO
2 flow rates of 3–7 L min
−1 completing a 15-min RedOx cycle. Through a temperature/pressure-swing operation within a reactor depicted in
Figure 11b, separate streams of O
2 and CO with almost 100% selectivity were produced. Also, a molar CO
2 conversion to CO of 83% together with ƞ
solar-to-fuel = 5.25% were obtained, which is around three times more than the previously reported values i.e., 1.73% on average and 3.53% at peak
[9].
Figure 5. (
a) Schematic representation of the solar reactor for carbon dioxide splitting, comprising a windowed cavity-receiver containing a ceria RPC with dual pore sizes (mm- and μm-sized pores); (
b) images of the front face of the solar reactor with the windowed aperture and its interior containing the octagonal ceria RPC structure insulated with a line of alumina
[30].
A volumetric, directly irradiated solar cavity receiver equipped with 5-ppi ceria RPC was directly irradiated in the temperature range of 950–1050 °C for syngas production and isothermal H
2O/CO
2 splitting
[31]. The ceria reduction was performed with methane (partial oxidation of methane), while the oxidation was completed by H
2O/CO
2 under the same operating temperature. Methane was employed to reduce the reduction temperature and, hence, the technical challenges associated with the high temperatures and the parasitic losses. Various operating parameters such as methane flow rate and reduction temperature were studied on the CO
2 conversion and, thus, syngas yield. It was shown that an increase in the methane flow rate and decrease in temperature resulted in carbon formation during methane cracking, while increasing the nonstoichiometry value (δ up to 0.38) led to greater syngas yields, with a maximum value of 8.08 mol of methane per kg of CeO
2. The material stability was reported to be high enough after 15 successive ceria RedOx cycles with the highest solar-to-fuel energy conversion efficiency of 5.22% and the energy upgrade factor in the range of 0.97–1.10. Nonetheless, further cyclic RedOx assessments are needed to justify the material stability.
Despite the significant effort that has been allocated to the development of various RedOx pairs, the cerium oxide and perovskite materials have been relatively more attractive. This is due to their potential for higher efficiency and durability over successive RedOx cycles. In a recent, unprecedented work, a nano perovskite-coated silicon carbide RPC with dual pore sizes of macro pores between struts (d
p = 2.54 mm) and µm-sized pores inside struts (d
p = 490 µm) was tested in an infrared furnace to assess the capability of the developed materials for CO
2 splitting
[4]. The porous structure was coated by the dip-coating method
[32] with ca. 15-mm thickness of La
0.6Ca
0.4Mn
0.6Al
0.4O
3±δ (LCMA) as active RedOx material. The LCMA-coated porous substrate could convert CO
2 to CO with a concentration of [CO%] = 3.2 in a controlled atmosphere of 10 vol.% CO
2 feed gas at 1050 °C after around 1.5 h of reduction reaction at 1240 °C. However, the XCT characterization revealed that the process distorted the RPC by coagulation of smaller pores within struts and some diffusion of LCMA into struts’ pores. As a complementary work
[11], three different pore sizes of porous substrates, i.e., 5, 12, and 75 ppi, were also investigated to assess the effects of pore architecture on CO
2 conversion efficiency.
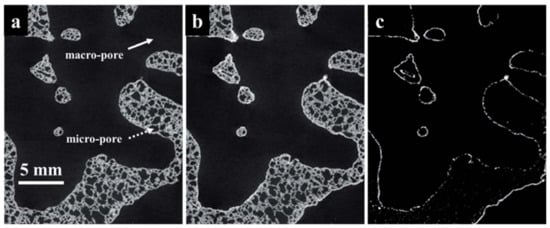
Figure 6 illustrates the 2D tomograms of 12 ppi sample (a) before; (b) after LCMA perovskite coating; (c) after tomograms registration showing the perovskite coating layer. Although the sample with pore size of 12 ppi possessed the most homogeneity of RedOx coating layer as much as the highest perovskite loading, 75 ppi porous sample with the smallest pore sizes delivered the highest CO yield of ca. 0.07 molg
−1 LCMA. The non-stoichiometry oxygen was also calculated to be δ = 0.4.
Figure 6. The 2D tomogram slices of a porous SiC sample with dual-scale pore sizes including macro pores between struts and µm-sized micropores inside struts; (
a) before; (
b) after LCMA perovskite coating; and (
c) after tomograms’ registration showing the perovskite mapping
[11].
Haeussler et al.
[5] tested a series of ceria RPCs with gradient pore sizes in the range of 10–60 ppi within a 1.5 kW
th directly irradiated solar reactor. The temperature-swing RedOx reactions were performed (TR: 1400–1450 °C and WS/CDS at 700–1100 °C) to produce pure H
2 or CO inside the same reactor (
Figure 7). Similarly, the results demonstrated that the degree of ceria reduction increases with decreasing of both the temperature and the operating pressure. Moreover, it was found that the oxidation rate could be improved (up to 9.3 mLg
−1 min
−1) by increasing the inlet CO
2 flow rate which is attributed to the increasing of the reactions driving force. A maximum η
solar-to-fuel of ~7.5% was also measured after 64 cycles with a high stability of the porous RedOx structures
[5].
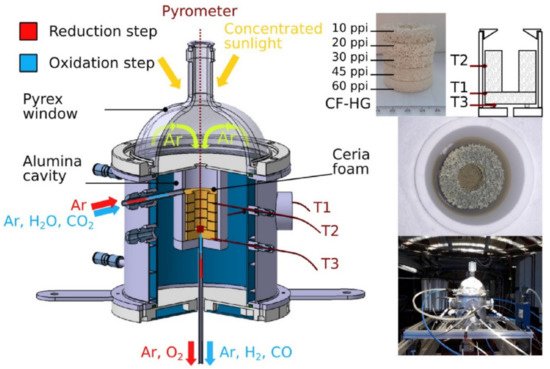
Figure 7. Schematic of the 1.5-kW
th directly irradiated solar reactor equipped with a series of ceria RPCs with 10–60-ppi gradient pore sizes used for H
2O or CO
2 splitting
[5].
Subsequent to the previous work, the La
0.5Sr
0.5Mn
0.9Mg
0.1O
3 (LSMMg)-coated ceria foam was tested for steam and carbon dioxide splitting
[1]. The results showed an improved reduction extent mainly due to the perovskite layer, but no improvement was observed in the re-oxidation and the fuel production rate compared to the pure uncoated ceria RPC. It was concluded that the use of LSMMg perovskite coating (~10-µm thickness) worked as a layer that hindered the oxidant gas (H
2O or CO
2) from accessing the reactive ceria, which possessed a higher re-oxidation capability. However, it was shown that by optimization of some reaction parameters such as pressure during the reduction cycle and the total gas flow rate as well as the oxidant molar fraction during the re-oxidation step the fuel production rate could be increased. This research on porous RedOx materials with coupled positive aspects of two different active materials showed a maximum η
solar-to-fuel = 5.3, which was very close to the value for the uncoated ceria foam (peak η
solar-to-fuel = 5.5). The role of this composite synthesis was synergistic oxygen release in the reduction cycle and resulted in a higher total fuel production relative to the uncoated ceria.