Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Naeh, A. Gestational Diabetes and Its Prevention. Encyclopedia. Available online: https://encyclopedia.pub/entry/17630 (accessed on 07 January 2026).
Naeh A. Gestational Diabetes and Its Prevention. Encyclopedia. Available at: https://encyclopedia.pub/entry/17630. Accessed January 07, 2026.
Naeh, Amir. "Gestational Diabetes and Its Prevention" Encyclopedia, https://encyclopedia.pub/entry/17630 (accessed January 07, 2026).
Naeh, A. (2021, December 29). Gestational Diabetes and Its Prevention. In Encyclopedia. https://encyclopedia.pub/entry/17630
Naeh, Amir. "Gestational Diabetes and Its Prevention." Encyclopedia. Web. 29 December, 2021.
Copy Citation
Gestational diabetes mellitus (GDM) complicates between 5 and 12% of pregnancies, with
associated maternal, fetal, and neonatal complications. The ideal screening and diagnostic criteria
to diagnose and treat GDM have not been established, and in recent years, significant research has been undertaken to identify a first-trimester biomarker that can predict GDM later in pregnancy, enable early intervention, and reduce GDM-related adverse outcomes. This review summarizes current data on first-trimester biomarkers, the advantages, and the
limitations.
gestational diabetes
biomarkers
early prediction
pregnancy complications
1. Predicting GDM by Maternal Risk Factors
Table 1. Clinical risk factors with corresponding odds ratios for gestational diabetes mellitus.
| Risk Factor | Odds Ratio |
|---|---|
| 1. Ethnicity: Asian, Middle Eastern, Hispanic, Latino, African American, and Indigenous | 2.32 [1] |
| 2. Maternal age ≥35 years | 3.54 [2] |
| 3. Pre-pregnancy BMI >25 kg/m2 | 2.14 [3] |
| 4. Polycystic ovary syndrome | 2.32 [4] |
| 5. GDM in a previous pregnancy | 5.9 [1] |
| 6. Previous delivery of macrocosmic baby (birth weight >4000 gr or >90th centile) | 1.54 [1] |
| 7. Family history of diabetes (1st-degree relative) | 1.36 [1] |
| 8. Multiple pregnancy | 1.13 [5] |
| 9. Assisted reproductive technology | 1.26 [6] |
BMI—body mass index and GDM—gestational diabetes mellitus.
2. Predicting GDM Using Individual Biomarkers
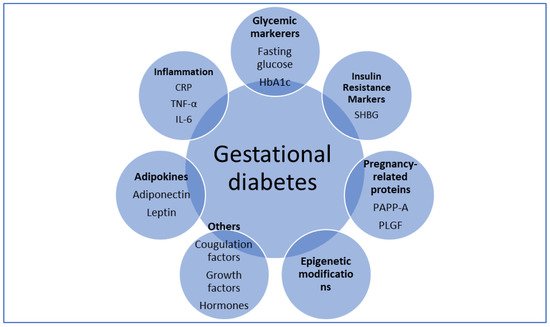
Figure 1. Overview of tested biomarkers. CRP—C-reactive protein; IL-6—interleukin 6; PAPP-A—pregnancy-associated plasma protein A; PLGF—placental growth factor; SHBG—sex hormone-binding globulin; and TNF-α—tumor necrosis factor α.
Table 2. Potential biomarkers for gestational diabetes mellitus.
| Biomarker | Function | Suggested Involvement in GDM Pathophysiology |
|---|---|---|
| Adiponectin | Modulation of glucose and fatty acid metabolism. Involvement in inflammation, apoptosis, and angiogenesis. | Low levels associated with decreased insulin sensitivity and GDM |
| Leptin | Regulation of energy balance and expenditure. Role in hormone regulation and immunity. | High leptin levels cause hyperinsulinemia and increase insulin resistance |
| PAPP-A | Increase bioavailability of IGF-1 and promotes somatic growth. Involvement in wound healing and bone remodelling. | Decreased levels contribute to an increase in insulin resistance |
| PLGF | Vascular endothelial growth factor-like protein. Role in angiogenesis and placentation. | High PLGF levels promote the abnormal vascular network in placentas of GDM pregnancies |
| TNF-α | Inflammatory cytokine involved in the regulation of immune cells, inflammation, and autoimmune diseases. | Increased levels impair insulin signalling and beta-cell function, leading to insulin resistance and GDM |
| CRP | Acute-phase reactant. Role in tissue injury, inflammation, and infection. | High levels associated with insulin resistance and systemic inflammation |
| IL-6 | Circulating inflammatory cytokine. Role in immune response regulation, inflammation, and hematopoiesis. | Increased secretion by adipocytes and placental cells, leading to a chronic inflammatory process and insulin resistance |
| SHBG | Glycoprotein that binds androgen and estrogen. | Decrease SHBG levels associated with hyperinsulinemia and GDM |
3. Predicting GDM by Early Glycemic Markers
3.1. Fasting Glucose
In 2010, the International Association of Diabetes and Pregnancy Study Group recommended different thresholds for diagnosis and classification of hyperglycemia in pregnancy based on the results of the HAPO study [7]. The panel recommended the use of first-trimester fasting glucose above 92 mg/dL to diagnose early GDM; however, this approach was criticized due to a lack of evidence [8][9]. Although not established as a diagnostic marker, several studies have demonstrated an association between first-trimester fasting glucose and GDM development in late pregnancy. Mashiah et al. showed strong, graded associations between fasting glucose levels in the first trimester and GDM development, macrosomia, and cesarean section [10]. Other studies have shown that elevated first-trimester fasting glucose levels have sensitivities of 47–75% and specificities of 52–77% for the prediction of GDM later in pregnancy [11][12], making the predictive value of fasting glucose similar to the risk factor of BMI [11]. Nevertheless, a definitive clinically useful cut-off for first-trimester fasting glucose has not been established and results show an insufficient predictive value for this marker.
3.2. HbA1c
HbA1c is used to estimate average blood glucose over the lifespan of a red blood cell (~120 days). HbA1c does not require fasting and is used in non-pregnant populations to diagnose and monitor both pre-diabetes and diabetes [13]. Several studies demonstrated that a first-trimester HbA1c in the pre-diabetic values (5.7–6.4%) is correlated with GDM manifestation in late pregnancy and associated with adverse pregnancy outcomes [14][15][16]. Although a significant risk factor for GDM, the low sensitivity of HbA1c in this range makes it a poor test to identify women who will develop GDM [15]. HbA1c levels are also subjected to pregnancy changes and possibly require pregnancy-specific reference ranges [17]. Finally, Osmundson et al. conducted a randomized control trial which showed that treatment of women with early (before 14 weeks’ gestation) pre-diabetic HbA1c (5.7–6.4%) did not reduce the risk of GDM later in pregnancy, except in non-obese women [18]. In summary, current evidence does not support the use of HbA1c as an effective early predictor for GDM.
4. Predicting GDM by Adipokines
4.1. Adiponectin
Adiponectin is a protein secreted primarily by the adipose tissue but also by the brain, skeletal muscle, and placenta [19]. Adiponectin augments insulin sensitivity and low levels of adiponectin are associated with obesity, T2DM, hypertension, and coronary artery disease [20][21]. In normal pregnancy, maternal adiponectin secretion progressively declines probably due to decreased insulin sensitivity [22].
Previous studies have demonstrated that low levels of adiponectin in the first trimester are associated with GDM development later in pregnancy [23][24][25]. A metanalysis by Iliodromiti et al. suggested that early pregnancy adiponectin levels have a moderate predictive value for GDM, similar to that of clinical risk factors [26]. In summary, adiponectin may play a role in the pathophysiology of GDM and has the potential for a promising predictive biomarker. Further research is needed to further establish the true value of adiponectin in GDM prediction.
4.2. Leptin
Leptin regulates energy intake, suppresses appetite, and enhances the insulin effect with both central and peripheral effects [27]. Leptin is also expressed by placental cells and levels increase up to three-folds in pregnancy likely due to placental secretion and an increase in fat tissue; however, its exact role in pregnancy remains unclear [28][29]. Evidence regarding the association between leptin levels and GDM is inconsistent, with some studies demonstrating higher leptin levels in women who subsequently develop GDM [30][31], whereas others showed no difference [32][33]. Future prospective studies are required to determine leptin predictive ability in GDM while adequately addressing the confounding influence of BMI and gestational weight gain on leptin levels in pregnancy.
5. Predicting GDM by Pregnancy-Related Proteins
5.1. Pregnancy Associated Plasma Protein A (PAPP-A)
PAPP-A is a metalloproteinase that increases the bioavailability of insulin-like growth factor 1 (IGF-1) by its cleavage from IGF binding protein-4 [34]. In pregnancy, PAPP-A is secreted by trophoblast cells and used as a first-trimester screening test for aneuploidy, as well as a predictor of placental disorders such as preeclampsia and fetal growth restriction [35]. It has been hypothesized that PAPP-A has a role in regulating glucose levels in pregnancy, with low levels associated with insulin resistance and GDM.
Nonetheless, previous studies have yielded conflicting results. A number of studies reported low first-trimester PAPP-A levels in women who eventually developed GDM [36][37][38][39]. Additionally, a large systematic review and meta-analysis by Donovan et al. concluded that women who are diagnosed with GDM have lower first-trimester levels of PAPP-A, even though a high degree of between-study heterogeneity was noted [40]. Other studies failed to demonstrate differences in PAPP-A levels in pregnancies with GDM compared to normal pregnancies [41][42] and Syngelaki et al. showed that the performance of screening for GDM by maternal factors was not improved by the addition of PAPP-A [43]. Future prospective studies are required to establish the clinical utility of this biomarker.
5.2. Placental Growth Factor (PLGF)
PLGF is an angiogenic protein highly expressed in the placenta [44]. PLGF is widely used in aneuploidy screening in the first trimester, with low levels associated with placental-mediated disorders, mainly preeclampsia and fetal growth restriction [45].
Several studies have shown that elevated PLGF levels in early pregnancy are associated with GDM development [46][47]. However, other studies have demonstrated no differences in PLGF levels between women who developed GDM and controls [48][49]. Additionally, a large prospective cohort study from the UK showed that even though early PLGF levels were higher in women with GDM, the addition of PLGF to a prediction model that includes maternal factors did not improve the predictive ability [43]. Therefore, current evidence does not support the use of PLGF as an effective biomarker for GDM.
5.3. First-Trimester Combined Test (FTCT)
The FTCT is an effective screening tool for fetal aneuploidy, which includes the combination of maternal age, ultrasound measurement of fetal nuchal translucency, and the serum markers-free β-human chorionic gonadotropin and PAPP-A. As an early, routinely implemented test, which includes pregnancy-related proteins, the FTCT has the potential to be used as a tool for the prediction of GDM. Visconti et al. showed that an FTCT result of <1:10,000 was significantly associated with GDM development later in pregnancy, but with low accuracy [50], and other studies failed to demonstrate this association [42][51]. Based on current evidence, it is unclear whether the use of low PAPP-A solely (in absolute levels or in multiples of the median) contributes to GDM prediction better than the entire FTCT.
6. Predicting GDM by Inflammatory Markers
6.1. Tumor Necrosis Factor-α (TNF-α)
TNF-α is an inflammatory cytokine that is produced by placental cells and has been suggested as a mediator for insulin resistance in pregnancy [52]. While some studies showed an association between elevated levels of TNF-α in the first trimester and GDM development later in pregnancy [53][54], others failed to demonstrate this effect [33][55]. Furthermore, adding TNF-α to a screening model did not improve the prediction of GDM over maternal clinical characteristics [56]. In summary, even though TNF-α probably has a role in the pathogenesis of insulin resistance and GDM, the actual predictive value of this biomarker is yet to be established.
6.2. C-Reactive Protein (CRP)
CRP is an acute-phase protein secreted and released in response to tissue injury, inflammation, and infection [57]. Evidence regarding the association between levels of CRP or high-sensitivity CRP and GDM is inconsistent, with multiple studies showing high first-trimester levels in women who eventually develop GDM [58][59][60][61] and some studies not [56][62]. A possible confounder is the fact that CRP levels in pregnancy correlate with BMI and a prospective study by Wolf et al. concluded that the association between increased CRP and GDM was attenuated when BMI was included in the model [63]; the same effect was reported by others as well [64][65]. A recent systematic review by Amirian et al. discussed conflicting evidence and concluded that more studies are needed for CRP to be used as an indicator for GDM [66].
6.3. Interleukin 6 (IL-6)
IL-6 is a circulating inflammatory cytokine secreted by adipocytes as well as by macrophages, endothelial cells, pancreatic cells, and placenta cells [67]. IL-6 is involved in the regulation of immune response regulation, inflammation, and hematopoiesis [68], but also has a significant role in obesity and insulin resistance [69]. A systematic review and meta-analysis by Wang et al. concluded that IL-6 is a strong predictor of developing T2DM [70].
Previous studies showed an association between high levels of IL-6 and GDM [71][72][73]; however, these studies were limited by the gestational age at which IL-6 levels were measured, the population assessed, and by controlling for confounders such as BMI. Other studies failed to demonstrate a difference in IL-6 levels between GDM and normal pregnancies [74][75]. Nevertheless, a study by Hassiakos et al. showed that first-trimester IL-6 levels were a significant predictor of GDM development later in pregnancy and adding IL-6 to a prediction model that included maternal characteristics yielded an improved prediction [76]. A systematic review by Amirian et al. concluded that IL-6 levels are significantly higher in pregnant women with GDM than in healthy pregnant women and therefore the evaluation of this marker as a GDM predictor can be investigated [77]. In summary, although IL-6 has the potential to be a good biomarker for GDM in the future as demonstrated in T2DM, current evidence does not support it as such. Larger prospective studies are needed to assess IL-6 function in GDM as well as to adjust for obesity as a confounder and to obtain serial IL-6 measurements for the identification of trimester-specific ranges.
7. Predicting GDM by Insulin Resistance Markers
SHBG
SHBG is a glycoprotein produced mainly by the liver, binds androgen and estrogen, and has an inverse relationship with insulin levels [78]. Low levels of SHBG prior to pregnancy [79][80] and in the first trimester [58][81][82] were found to be correlated with GDM development later in pregnancy. Nanda et al. showed that adding SHBG to a clinical risk prediction model improved its accuracy [83]. However, in other studies, this association was not found [84] or was no longer significant after adjusting for BMI, ethnicity, and family history [62]. Hence, even though SHBG is a very promising marker for early diagnosis of GDM, with even pre-pregnancy predictability, further studies are required to establish its role.
References
- Farrar, D.; Simmonds, M.; Bryant, M.; Lawlor, D.A.; Dunne, F.; Tuffnell, D.; Sheldon, T.A. Risk factor screening to identify women requiring oral glucose tolerance testing to diagnose gestational diabetes: A systematic review and meta-analysis and analysis of two pregnancy cohorts. PLoS ONE 2017, 12, e0175288.
- Li, Y.; Ren, X.; He, L.; Li, J.; Zhang, S.; Chen, W. Maternal age and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of over 120 million participants. Diabetes Res. Clin. Pract. 2020, 162, 108044.
- Chu, S.Y.; Callaghan, W.M.; Kim, S.Y.; Schmid, C.H.; Lau, J.; England, L.J.; Dietz, P.M. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007, 30, 2070–2076.
- Roos, N.; Kieler, H.; Sahlin, L.; Ekman-Ordeberg, G.; Falconer, H.; Stephansson, O. Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: Population based cohort study. BMJ 2011, 343, d6309.
- Hiersch, L.; Berger, H.; Okby, R.; Ray, J.G.; Geary, M.; Mcdonald, S.D.; Murry-Davis, B.; Riddell, C.; Halperin, I.; Hasan, H.; et al. Incidence and risk factors for gestational diabetes mellitus in twin versus singleton pregnancies. Arch. Gynecol. Obstet. 2018, 298, 579–587.
- Wang, Y.A.; Nikravan, R.; Smith, H.C.; Sullivan, E.A. Higher prevalence of gestational diabetes mellitus following assisted reproduction technology treatment. Hum. Reprod. Oxf. Engl. 2013, 28, 2554–2561.
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel; Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; de Leiva, A.; Hod, M.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682.
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S13–S27.
- Hillier, T.A.; Pedula, K.L.; Ogasawara, K.K.; Vesco, K.K.; Oshiro, C.E.S.; Lubarsky, S.L.; Van Marter, J. A Pragmatic, Randomized Clinical Trial of Gestational Diabetes Screening. N. Engl. J. Med. 2021, 384, 895–904.
- Riskin-Mashiah, S.; Younes, G.; Damti, A.; Auslender, R. First-trimester fasting hyperglycemia and adverse pregnancy outcomes. Diabetes Care 2009, 32, 1639–1643.
- Riskin-Mashiah, S.; Damti, A.; Younes, G.; Auslender, R. First trimester fasting hyperglycemia as a predictor for the development of gestational diabetes mellitus. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 152, 163–167.
- Yeral, M.I.; Ozgu-Erdinc, A.S.; Uygur, D.; Seckin, K.D.; Karsli, M.F.; Danisman, A.N. Prediction of gestational diabetes mellitus in the first trimester, comparison of fasting plasma glucose, two-step and one-step methods: A prospective randomized controlled trial. Endocrine 2014, 46, 512–518.
- American Diabetes Association. Standards of Medical Care in Diabetes-2016: Summary of Revisions. Diabetes Care 2016, 39 (Suppl. 1), S4–S5.
- Fong, A.; Serra, A.E.; Gabby, L.; Wing, D.A.; Berkowitz, K.M. Use of hemoglobin A1c as an early predictor of gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2014, 211, 641.e1–641.e7.
- Osmundson, S.S.; Zhao, B.S.; Kunz, L.; Wang, E.; Popat, R.; Nimbal, V.C.; Palaniappan, L.P. First Trimester Hemoglobin A1c Prediction of Gestational Diabetes. Am. J. Perinatol. 2016, 33, 977–982.
- Hughes, R.C.E.; Moore, M.P.; Gullam, J.E.; Mohamed, K.; Rowan, J. An early pregnancy HbA1c ≥5.9% (41 mmol/mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes. Diabetes Care 2014, 37, 2953–2959.
- Hughes, R.C.E.; Rowan, J.; Florkowski, C.M. Is There a Role for HbA1c in Pregnancy? Curr. Diabetes Rep. 2016, 16, 5.
- Osmundson, S.S.; Norton, M.E.; El-Sayed, Y.Y.; Carter, S.; Faig, J.C.; Kitzmiller, J.L. Early Screening and Treatment of Women with Prediabetes: A Randomized Controlled Trial. Am. J. Perinatol. 2016, 33, 172–179.
- Chen, J.; Tan, B.; Karteris, E.; Zervou, S.; Digby, J.; Hillhouse, E.W.; Vatish, M.; Randeva, H.S. Secretion of adiponectin by human placenta: Differential modulation of adiponectin and its receptors by cytokines. Diabetologia 2006, 49, 1292–1302.
- Chandran, M.; Phillips, S.A.; Ciaraldi, T.; Henry, R.R. Adiponectin: More than just another fat cell hormone? Diabetes Care 2003, 26, 2442–2450.
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935.
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 2010, 316, 129–139.
- Ferreira, A.F.A.; Rezende, J.C.; Vaikousi, E.; Akolekar, R.; Nicolaides, K.H. Maternal serum visfatin at 11-13 weeks of gestation in gestational diabetes mellitus. Clin. Chem. 2011, 57, 609–613.
- Lain, K.Y.; Daftary, A.R.; Ness, R.B.; Roberts, J.M. First trimester adipocytokine concentrations and risk of developing gestational diabetes later in pregnancy. Clin. Endocrinol. 2008, 69, 407–411.
- Lacroix, M.; Battista, M.-C.; Doyon, M.; Ménard, J.; Ardilouze, J.-L.; Perron, P.; Hivert, M.-F. Lower adiponectin levels at first trimester of pregnancy are associated with increased insulin resistance and higher risk of developing gestational diabetes mellitus. Diabetes Care 2013, 36, 1577–1583.
- Iliodromiti, S.; Sassarini, J.; Kelsey, T.W.; Lindsay, R.S.; Sattar, N.; Nelson, S.M. Accuracy of circulating adiponectin for predicting gestational diabetes: A systematic review and meta-analysis. Diabetologia 2016, 59, 692–699.
- Triantafyllou, G.A.; Paschou, S.A.; Mantzoros, C.S. Leptin and Hormones: Energy Homeostasis. Endocrinol. Metab. Clin. N. Am. 2016, 45, 633–645.
- Fattah, C.; Barry, S.; O’connor, N.; Farah, N.; Stuart, B.; Turner, M.J. Maternal leptin and body composition in the first trimester of pregnancy. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2011, 27, 263–266.
- Briana, D.D.; Malamitsi-Puchner, A. Reviews: Adipocytokines in normal and complicated pregnancies. Reprod. Sci. Thousand Oaks Calif. 2009, 16, 921–937.
- Bao, W.; Baecker, A.; Song, Y.; Kiely, M.; Liu, S.; Zhang, C. Adipokine levels during the first or early second trimester of pregnancy and subsequent risk of gestational diabetes mellitus: A systematic review. Metabolism 2015, 64, 756–764.
- Sommer, C.; Jenum, A.K.; Waage, C.W.; Mørkrid, K.; Sletner, L.; Birkeland, K.I. Ethnic differences in BMI, subcutaneous fat, and serum leptin levels during and after pregnancy and risk of gestational diabetes. Eur. J. Endocrinol. 2015, 172, 649–656.
- Maple-Brown, L.; Ye, C.; Hanley, A.J.; Connelly, P.W.; Sermer, M.; Zinman, B.; Retnakaran, R. Maternal pregravid weight is the primary determinant of serum leptin and its metabolic associations in pregnancy, irrespective of gestational glucose tolerance status. J. Clin. Endocrinol. Metab. 2012, 97, 4148–4155.
- Georgiou, H.M.; Lappas, M.; Georgiou, G.M.; Marita, A.; Bryant, V.J.; Hiscock, R.; Permezel, M.; Khalil, Z.; Rice, G.E. Screening for biomarkers predictive of gestational diabetes mellitus. Acta Diabetol. 2008, 45, 157–165.
- Boldt, H.B.; Conover, C.A. Pregnancy-associated plasma protein-A (PAPP-A): A local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm. IGF Res. 2007, 17, 10–18.
- Dugoff, L.; Hobbins, J.C.; Malone, F.D.; Porter, T.F.; Luthy, D.; Comstock, C.H.; Hankins, G.; Berkowitz, R.L.; Merkatz, I.; Craigo, S.D.; et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: A population-based screening study (the FASTER Trial). Am. J. Obstet. Gynecol. 2004, 191, 1446–1451.
- Ren, Z.; Zhe, D.; Li, Z.; Sun, X.-P.; Yang, K.; Lin, L. Study on the correlation and predictive value of serum pregnancy-associated plasma protein A, triglyceride and serum 25-hydroxyvitamin D levels with gestational diabetes mellitus. World J. Clin. Cases 2020, 8, 864–873.
- Beneventi, F.; Simonetta, M.; Lovati, E.; Albonico, G.; Tinelli, C.; Locatelli, E.; Spinillo, A. First trimester pregnancy-associated plasma protein-A in pregnancies complicated by subsequent gestational diabetes. Prenat. Diagn. 2011, 31, 523–528.
- Ramezani, S.; Doulabi, M.A.; Saqhafi, H.; Alipoor, M. Prediction of Gestational Diabetes by Measuring the Levels of Pregnancy Associated Plasma Protein-A (PAPP-A) During Gestation Weeks 11-14. J. Reprod. Infertil. 2020, 21, 130–137.
- Wells, G.; Bleicher, K.; Han, X.; McShane, M.; Chan, Y.F.; Bartlett, A.; White, C.; Lau, S.M. Maternal Diabetes, Large-for-Gestational-Age Births, and First Trimester Pregnancy-Associated Plasma Protein-A. J. Clin. Endocrinol. Metab. 2015, 100, 2372–2379.
- Donovan, B.M.; Nidey, N.L.; Jasper, E.A.; Robinson, J.G.; Bao, W.; Saftlas, A.F.; Ryckman, K.K. First trimester prenatal screening biomarkers and gestational diabetes mellitus: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0201319.
- Husslein, H.; Lausegger, F.; Leipold, H.; Worda, C. Association between pregnancy-associated plasma protein-A and gestational diabetes requiring insulin treatment at 11-14 weeks of gestation. J. Matern.-Fetal Neonatal Med. 2012, 25, 2230–2233.
- Savvidou, M.D.; Syngelaki, A.; Muhaisen, M.; Emelyanenko, E.; Nicolaides, K.H. First trimester maternal serum free β-human chorionic gonadotropin and pregnancy-associated plasma protein A in pregnancies complicated by diabetes mellitus. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 410–416.
- Syngelaki, A.; Kotecha, R.; Pastides, A.; Wright, A.; Nicolaides, K.H. First-trimester biochemical markers of placentation in screening for gestational diabetes mellitus. Metabolism 2015, 64, 1485–1489.
- Chau, K.; Hennessy, A.; Makris, A. Placental growth factor and pre-eclampsia. J. Hum. Hypertens. 2017, 31, 782–786.
- O’Gorman, N.; Wright, D.; Syngelaki, A.; Akolekar, R.; Wright, A.; Poon, L.C.; Nicolaides, K.H. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks gestation. Am. J. Obstet. Gynecol. 2016, 214, 103.e1–103.e12.
- Eleftheriades, M.; Papastefanou, I.; Lambrinoudaki, I.; Kappou, D.; Lavranos, D.; Akalestos, A.; Souka, A.P.; Pervanidou, P.; Hassiakos, D.; Chrousos, G.P. Elevated placental growth factor concentrations at 11–14 weeks of gestation to predict gestational diabetes mellitus. Metabolism 2014, 63, 1419–1425.
- Gorkem, U.; Togrul, C.; Arslan, E. Relationship between elevated serum level of placental growth factor and status of gestational diabetes mellitus. J. Matern.-Fetal Neonatal Med. 2020, 33, 4159–4163.
- Mosimann, B.; Amylidi, S.; Risch, L.; Wiedemann, U.; Surbek, D.; Baumann, M.; Stettler, C.; Raio, L. First-Trimester Placental Growth Factor in Screening for Gestational Diabetes. Fetal Diagn. Ther. 2016, 39, 287–291.
- Maymon, R.; Meiri, H.; Svirski, R.; Weiner, E.; Cuckle, H. Maternal serum screening marker levels in twin pregnancies affected by gestational diabetes. Arch. Gynecol. Obstet. 2019, 299, 655–663.
- Visconti, F.; Quaresima, P.; Chiefari, E.; Caroleo, P.; Arcidiacono, B.; Puccio, L.; Mirabelli, M.; Foti, D.P.; Di Carlo, C.; Vero, R.; et al. First Trimester Combined Test (FTCT) as a Predictor of Gestational Diabetes Mellitus. Int. J. Environ. Res. Public Health 2019, 16, 3654.
- Tul, N.; Pusenjak, S.; Osredkar, J.; Spencer, K.; Novak-Antolic, Z. Predicting complications of pregnancy with first-trimester maternal serum free-betahCG, PAPP-A and inhibin-A. Prenat. Diagn. 2003, 23, 990–996.
- Desoye, G.; Hauguel-de Mouzon, S. The human placenta in gestational diabetes mellitus. The insulin and cytokine network. Diabetes Care 2007, 30 (Suppl. 2), S120–S126.
- Xu, J.; Zhao, Y.H.; Chen, Y.P.; Yuan, X.L.; Wang, J.; Zhu, H.; Lu, C.M. Maternal circulating concentrations of tumor necrosis factor-alpha, leptin, and adiponectin in gestational diabetes mellitus: A systematic review and meta-analysis. Sci. World J. 2014, 2014, 926932.
- Gao, X.; Yang, H.; Zhao, Y. Variations of tumor necrosis factor-alpha, leptin and adiponectin in mid-trimester of gestational diabetes mellitus. Chin. Med. J. 2008, 121, 701–705.
- Guillemette, L.; Lacroix, M.; Battista, M.-C.; Doyon, M.; Moreau, J.; Ménard, J.; Ardilouze, J.-L.; Perron, P.; Hivert, M.-F. TNFα dynamics during the oral glucose tolerance test vary according to the level of insulin resistance in pregnant women. J. Clin. Endocrinol. Metab. 2014, 99, 1862–1869.
- Syngelaki, A.; Visser, G.H.A.; Krithinakis, K.; Wright, A.; Nicolaides, K.H. First trimester screening for gestational diabetes mellitus by maternal factors and markers of inflammation. Metabolism 2016, 65, 131–137.
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol. 2018, 9, 754.
- Maged, A.M.; Moety, G.A.F.; Mostafa, W.A.; Hamed, D.A. Comparative study between different biomarkers for early prediction of gestational diabetes mellitus. J. Matern.-Fetal Neonatal Med. 2014, 27, 1108–1112.
- Ozgu-Erdinc, A.S.; Yilmaz, S.; Yeral, M.I.; Seckin, K.D.; Erkaya, S.; Danisman, A.N. Prediction of gestational diabetes mellitus in the first trimester: Comparison of C-reactive protein, fasting plasma glucose, insulin and insulin sensitivity indices. J. Matern.-Fetal Neonatal Med. 2015, 28, 1957–1962.
- Alamolhoda, S.H.; Yazdkhasti, M.; Namdari, M.; Zakariayi, S.J.; Mirabi, P. Association between C-reactive protein and gestational diabetes: A prospective study. J. Obstet. Gynaecol. 2020, 40, 349–353.
- Alyas, S.; Roohi, N.; Ashraf, S.; Ilyas, S.; Ilyas, A. Early pregnancy biochemical markers of placentation for screening of gestational diabetes mellitus (GDM). Diabetes Metab. Syndr. 2019, 13, 2353–2356.
- Corcoran, S.M.; Achamallah, N.; Loughlin, J.O.; Stafford, P.; Dicker, P.; Malone, F.D.; Breathnach, F. First trimester serum biomarkers to predict gestational diabetes in a high-risk cohort: Striving for clinically useful thresholds. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 222, 7–12.
- Wolf, M.; Sandler, L.; Hsu, K.; Vossen-Smirnakis, K.; Ecker, J.L.; Thadhani, R. First-trimester C-reactive protein and subsequent gestational diabetes. Diabetes Care 2003, 26, 819–824.
- Retnakaran, R.; Hanley, A.J.G.; Raif, N.; Connelly, P.W.; Sermer, M.; Zinman, B. C-reactive protein and gestational diabetes: The central role of maternal obesity. J. Clin. Endocrinol. Metab. 2003, 88, 3507–3512.
- Berggren, E.K.; Roeder, H.A.; Boggess, K.A.; Moss, K.; Offenbacher, S.; Campbell, E.; Grotegut, C.A. First-trimester maternal serum C-reactive protein as a predictor of third-trimester impaired glucose tolerance. Reprod. Sci. Thousand Oaks Calif. 2015, 22, 90–93.
- Amirian, A.; Rahnemaei, F.A.; Abdi, F. Role of C-reactive Protein(CRP) or high-sensitivity CRP in predicting gestational diabetes Mellitus: Systematic review. Diabetes Metab. Syndr. 2020, 14, 229–236.
- Van Snick, J. Interleukin-6: An overview. Annu. Rev. Immunol. 1990, 8, 253–278.
- Jordan, S.C.; Choi, J.; Kim, I.; Wu, G.; Toyoda, M.; Shin, B.; Vo, A. Interleukin-6, A Cytokine Critical to Mediation of Inflammation, Autoimmunity and Allograft Rejection: Therapeutic Implications of IL-6 Receptor Blockade. Transplantation 2017, 101, 32–44.
- Hoene, M.; Weigert, C. The role of interleukin-6 in insulin resistance, body fat distribution and energy balance. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2008, 9, 20–29.
- Wang, X.; Bao, W.; Liu, J.; Ouyang, Y.-Y.; Wang, D.; Rong, S.; Xiao, X.; Shan, Z.-L.; Zhang, Y.; Yao, P.; et al. Inflammatory markers and risk of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2013, 36, 166–175.
- Morisset, A.-S.; Dubé, M.-C.; Côté, J.A.; Robitaille, J.; Weisnagel, S.J.; Tchernof, A. Circulating interleukin-6 concentrations during and after gestational diabetes mellitus. Acta Obstet. Gynecol. Scand. 2011, 90, 524–530.
- Siddiqui, S.; Waghdhare, S.; Goel, C.; Panda, M.; Soneja, H.; Sundar, J.; Banerjee, M.; Jha, S.; Dubey, S. Augmentation of IL-6 production contributes to development of gestational diabetes mellitus: An Indian study. Diabetes Metab. Syndr. 2019, 13, 895–899.
- Abell, S.K.; Shorakae, S.; Harrison, C.L.; Hiam, D.; Moreno-Asso, A.; Stepto, N.K.; De Courten, B.; Teede, H.J. The association between dysregulated adipocytokines in early pregnancy and development of gestational diabetes. Diabetes Metab. Res. Rev. 2017, 33, e2926.
- Braga, F.O.; Negrato, C.A.; Matta, M.D.F.B.D.; Carneiro, J.R.I.; Gomes, M.B. Relationship between inflammatory markers, glycated hemoglobin and placental weight on fetal outcomes in women with gestational diabetes. Arch. Endocrinol. Metab. 2019, 63, 22–29.
- Šimják, P.; Cinkajzlová, A.; Anderlová, K.; Kloučková, J.; Kratochvílová, H.; Lacinová, Z.; Kaválková, P.; Krejčí, H.; Mráz, M.; Pařízek, A.; et al. Changes in plasma concentrations and mRNA expression of hepatokines fetuin A, fetuin B and FGF21 in physiological pregnancy and gestational diabetes mellitus. Physiol. Res. 2018, 67, S531–S542.
- Hassiakos, D.; Eleftheriades, M.; Papastefanou, I.; Lambrinoudaki, I.; Kappou, D.; Lavranos, D.; Akalestos, A.; Aravantinos, L.; Pervanidou, P.; Chrousos, G. Increased Maternal Serum Interleukin-6 Concentrations at 11 to 14 Weeks of Gestation in Low Risk Pregnancies Complicated with Gestational Diabetes Mellitus: Development of a Prediction Model. Horm. Metab. Res. Horm. Stoffwechs. Horm. Metab. 2016, 48, 35–41.
- Amirian, A.; Mahani, M.B.; Abdi, F. Role of interleukin-6 (IL-6) in predicting gestational diabetes mellitus. Obstet. Gynecol. Sci. 2020, 63, 407–416.
- Wallace, I.R.; McKinley, M.C.; Bell, P.M.; Hunter, S.J. Sex hormone binding globulin and insulin resistance. Clin. Endocrinol. 2013, 78, 321–329.
- Hedderson, M.M.; Xu, F.; Darbinian, J.A.; Quesenberry, C.P.; Sridhar, S.; Kim, C.; Gunderson, E.P.; Ferrara, A. Prepregnancy SHBG concentrations and risk for subsequently developing gestational diabetes mellitus. Diabetes Care 2014, 37, 1296–1303.
- Veltman-Verhulst, S.M.; van Haeften, T.W.; Eijkemans, M.J.C.; de Valk, H.W.; Fauser, B.C.J.M.; Goverde, A.J. Sex hormone-binding globulin concentrations before conception as a predictor for gestational diabetes in women with polycystic ovary syndrome. Hum. Reprod. Oxf. Engl. 2010, 25, 3123–3128.
- Caglar, G.S.; Ozdemir, E.D.U.; Cengiz, S.D.; Demirtaş, S. Sex-hormone-binding globulin early in pregnancy for the prediction of severe gestational diabetes mellitus and related complications. J. Obstet. Gynaecol. Res. 2012, 38, 1286–1293.
- Smirnakis, K.V.; Plati, A.; Wolf, M.; Thadhani, R.; Ecker, J.L. Predicting gestational diabetes: Choosing the optimal early serum marker. Am. J. Obstet. Gynecol. 2007, 196, 410.e1-6.
- Nanda, S.; Savvidou, M.; Syngelaki, A.; Akolekar, R.; Nicolaides, K.H. Prediction of gestational diabetes mellitus by maternal factors and biomarkers at 11 to 13 weeks. Prenat. Diagn. 2011, 31, 135–141.
- McElduff, A.; Hitchman, R.; McElduff, P. Is sex hormone-binding globulin associated with glucose tolerance? Diabet. Med. J. Br. Diabet. Assoc. 2006, 23, 306–312.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
30 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




