
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Danzan Mansorunov | + 2513 word(s) | 2513 | 2021-12-23 06:41:43 | | | |
| 2 | Nora Tang | Meta information modification | 2513 | 2021-12-27 09:15:21 | | | | |
| 3 | Nora Tang | Meta information modification | 2513 | 2021-12-29 10:11:34 | | |
Video Upload Options
To increase the effectiveness of anticancer therapy based on immune checkpoint (IC) inhibition, some ICs are being investigated in addition to those used in clinic. Increased expression of the most studied ICs—PD-L1, B7-H3, and B7-H4—is associated with poor survival; their inhibition is clinically significant. Expression of IDO1, CD155, and ADAM17 is also associated with poor survival, including gastric cancer (GC). The available data indicate that CD155 and ADAM17 are promising targets for immune therapy. However, the clinical trials of anti-IDO1 antibodies have been unsatisfactory. Expression of Galectin-3 and -9, CEACAM1 and Siglec-15 demonstrates a contradictory relationship with patient survival. In conclusion, in many cases it is important to analyze the expression of other participants of the immune response besides target IC. The PD-L1, B7-H3, B7-H4, IDO1 and ADAM17 may be considered as candidates for prognosis markers for GC patient survival.
1. Introduction
2. Immune Checkpoints as Biomarkers of GC
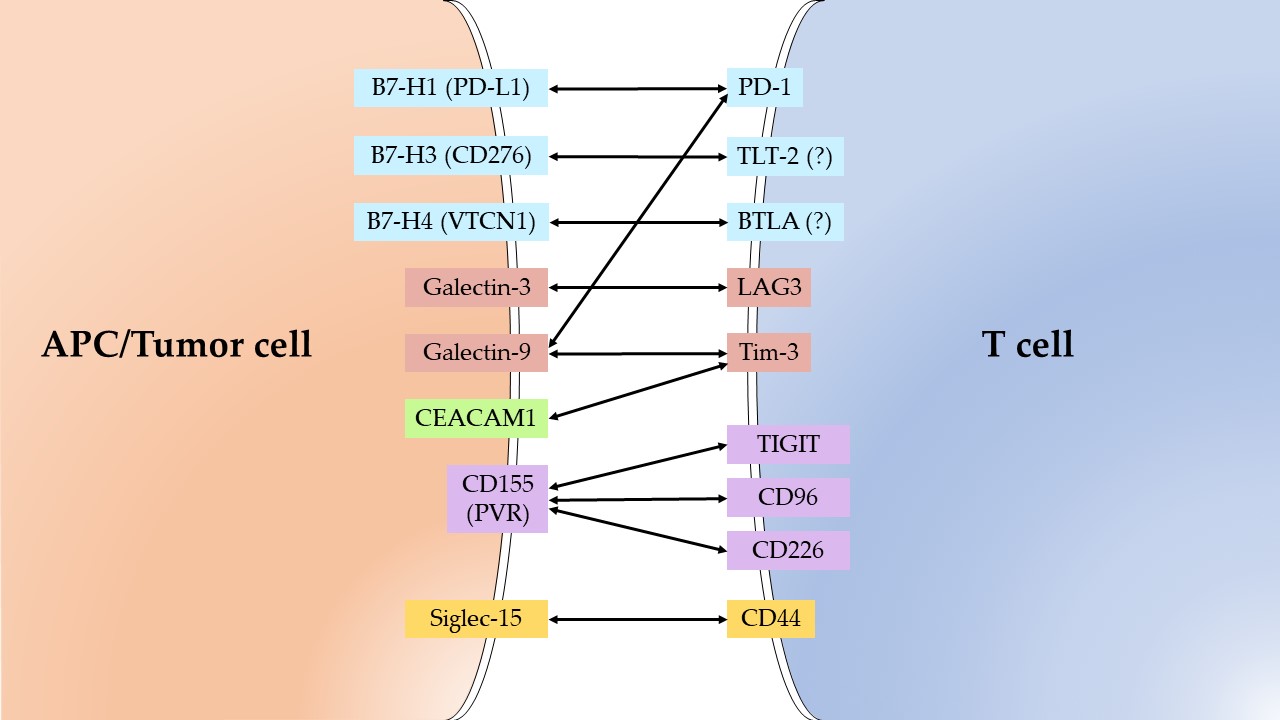
The analyzed IC ligands, expressed in tumors, and their receptors on T-cells are presented on Figure 1.
2.1. PD-L1
2.2. B7-H3
2.3. B7-H4
2.4. Galectin-3
2.5. Galectin-9
2.6. IDO1
2.7. CEACAM1,CD155 and Siglec-15
2.8. ADAM17
3. Conclusions
References
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362.
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856.
- Bolandi, N.; Derakhshani, A.; Hemmat, N.; Baghbanzadeh, A.; Asadzadeh, Z.; Afrashteh Nour, M.; Brunetti, O.; Bernardini, R.; Silvestris, N.; Baradaran, B. The Positive and Negative Immunoregulatory Role of B7 Family: Promising Novel Targets in Gastric Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 10719.
- Cao, Y.; Wang, X.; Jin, T.; Tian, Y.; Dai, C.; Widarma, C.; Song, R.; Xu, F. Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy. Signal Transduct. Target. Ther. 2020, 5, 250.
- Zhang, Y.; Zheng, J. Functions of Immune Checkpoint Molecules Beyond Immune Evasion. Adv. Exp. Med. Biol. 2020, 1248, 201–226.
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249.
- Etemadi, A.; Safiri, S.; Sepanlou, S.G.; Ikuta, K.; Bisignano, C.; Shakeri, R.; Amani, M.; Fitzmaurice, C.; Nixon, M.; Abbasi, N.; et al. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 42–54.
- Roviello, G.; Corona, S.P.; D’Angelo, A.; Rosellini, P.; Nobili, S.; Mini, E. Immune Checkpoint Inhibitors in Pre-Treated Gastric Cancer Patients: Results from a Literature-Based Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 448.
- Qiu, Z.; Du, Y. Clinicopathological and prognostic significance of programmed death ligant-1 expression in gastric cancer: A meta-analysis. J. Gastrointest. Oncol. 2021, 12, 112–120.
- Gu, L.; Chen, M.; Guo, D.; Zhu, H.; Zhang, W.; Pan, J.; Zhong, X.; Li, X.; Qian, H.; Wang, X. PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0182692.
- Zhang, M.; Dong, Y.; Liu, H.; Wang, Y.; Zhao, S.; Xuan, Q.; Wang, Y.; Zhang, Q. The clinicopathological and prognostic significance of PD-L1 expression in gastric cancer: A meta-analysis of 10 studies with 1,901 patients. Sci. Rep. 2016, 6, 37933.
- Feng, X.-S.; Wang, X.-S.; Wang, Y.-F.; Hu, X.-C.; Yan, J.-Q.; Wang, W.; Yang, R.-J.; Feng, Y.-Y.; Gao, S.-G.; Liu, Y.-X.; et al. Prognostic significance of PD-L1 expression in patients with gastric cancer in East Asia: A meta-analysis. Onco. Targets. Ther. 2016, 9, 2649.
- Xu, F.; Feng, G.; Zhao, H.; Liu, F.; Xu, L.; Wang, Q.; An, G. Clinicopathologic Significance and Prognostic Value of B7 Homolog 1 in Gastric Cancer: A Systematic Review and Meta-Analysis. Medicine 2015, 94, e1911.
- Shigemori, T.; Toiyama, Y.; Okugawa, Y.; Yamamoto, A.; Yin, C.; Narumi, A.; Ichikawa, T.; Ide, S.; Shimura, T.; Fujikawa, H.; et al. Soluble PD-L1 Expression in Circulation as a Predictive Marker for Recurrence and Prognosis in Gastric Cancer: Direct Comparison of the Clinical Burden Between Tissue and Serum PD-L1 Expression. Ann. Surg. Oncol. 2019, 26, 876–883.
- Ito, S.; Fukagawa, T.; Noda, M.; Hu, Q.; Nambara, S.; Shimizu, D.; Kuroda, Y.; Eguchi, H.; Masuda, T.; Sato, T.; et al. Prognostic Impact of Immune-Related Gene Expression in Preoperative Peripheral Blood from Gastric Cancer Patients. Ann. Surg. Oncol. 2018, 25, 3755–3763.
- Zhan, S.; Liu, Z.; Zhang, M.; Guo, T.; Quan, Q.; Huang, L.; Guo, L.; Cao, L.; Zhang, X. Overexpression of B7-H3 in α-SMA-Positive Fibroblasts Is Associated with Cancer Progression and Survival in Gastric Adenocarcinomas. Front. Oncol. 2020, 9, 1466.
- Arigami, T.; Uenosono, Y.; Hirata, M.; Yanagita, S.; Ishigami, S.; Natsugoe, S. B7-H3 expression in gastric cancer: A novel molecular blood marker for detecting circulating tumor cells. Cancer Sci. 2011, 102, 1019–1024.
- Cui, Y.; Li, Z. B7-H4 is Predictive of Poor Prognosis in Patients with Gastric Cancer. Med. Sci. Monit. 2016, 22, 4233–4237.
- Jiang, J.; Zhu, Y.; Wu, C.; Shen, Y.; Wei, W.; Chen, L.; Zheng, X.; Sun, J.; Lu, B.; Zhang, X. Tumor expression of B7-H4 predicts poor survival of patients suffering from gastric cancer. Cancer Immunol. Immunother. 2010, 59, 1707–1714.
- Arigami, T.; Uenosono, Y.; Ishigami, S.; Hagihara, T.; Haraguchi, N.; Natsugoe, S. Clinical significance of the B7-H4 coregulatory molecule as a novel prognostic marker in gastric cancer. World J. Surg. 2011, 35, 2051–2057.
- Arigami, T.; Uenosono, Y.; Hirata, M.; Hagihara, T.; Yanagita, S.; Ishigami, S.; Natsugoe, S. Expression of B7-H4 in blood of patients with gastric cancer predicts tumor progression and prognosis. J. Surg. Oncol. 2010, 102, 748–752.
- Shi, H.; Ji, M.; Wu, J.; Zhou, Q.; Li, X.; Li, Z.; Zheng, X.; Xu, B.; Zhao, W.; Wu, C.; et al. Serum B7-H4 expression is a significant prognostic indicator for patients with gastric cancer. World J. Surg. Oncol. 2014, 12, 188.
- Long, B.; Yu, Z.; Zhou, H.; Ma, Z.; Ren, Y.; Zhan, H.; Li, L.; Cao, H.; Jiao, Z. Clinical characteristics and prognostic significance of galectins for patients with gastric cancer: A meta-analysis. Int. J. Surg. 2018, 56, 242–249.
- Okada, K.; Shimura, T.; Suehiro, T.; Mochiki, E.; Kuwano, H. Reduced galectin-3 expression is an indicator of unfavorable prognosis in gastric cancer. Anticancer Res. 2006, 26, 1369–1376.
- Kim, S.J.; Kim, D.C.; Kim, M.C.; Jung, G.J.; Kim, K.H.; Jang, J.S.; Kwon, H.C.; Kim, Y.M.; Jeong, J.S. Fascin expression is related to poor survival in gastric cancer. Pathol. Int. 2012, 62, 777–784.
- Li, Y. Serum Galectin-3 as a Potential Marker for Gastric Cancer. Med. Sci. Monit. 2015, 21, 755–760.
- Choi, S.I.; Seo, K.W.; Kook, M.C.; Kim, C.G.; Kim, Y.W.; Cho, S.J. Prognostic value of tumoral expression of galectin-9 in gastric cancer. Turkish J. Gastroenterol. 2017, 28, 166–170.
- Jiang, J.; Jin, M.-S.; Kong, F.; Cao, D.; Ma, H.-X.; Jia, Z.; Wang, Y.-P.; Suo, J.; Cao, X. Decreased galectin-9 and increased Tim-3 expression are related to poor prognosis in gastric cancer. PLoS ONE 2013, 8, e81799.
- Liu, H.; Shen, Z.; Wang, Z.; Wang, X.; Zhang, H.; Qin, J.; Qin, X.; Xu, J.; Sun, Y. Increased expression of IDO associates with poor postoperative clinical outcome of patients with gastric adenocarcinoma. Sci. Rep. 2016, 6, 21319.
- Nishi, M.; Yoshikawa, K.; Higashijima, J.; Tokunaga, T.; Kashihara, H.; Takasu, C.; Ishikawa, D.; Wada, Y.; Shimada, M. The Impact of Indoleamine 2,3-dioxygenase (IDO) Expression on Stage III Gastric Cancer. Anticancer Res. 2018, 38, 3387–3392.
- Ni, P.; Yu, M.; Zhang, R.; He, M.; Wang, H.; Chen, S.; Duan, G. Prognostic Significance of ADAM17 for Gastric Cancer Survival: A Meta-Analysis. Medicina 2020, 56, 322.
- Zhang, T.; Zhu, W.; Huang, M.; Fan, R.; Chen, X. Prognostic value of ADAM17 in human gastric cancer. Med. Oncol. 2012, 29, 2684–2690.
- Shou, Z.-X.; Jin, X.; Zhao, Z.-S. Upregulated Expression of ADAM17 Is a Prognostic Marker for Patients with Gastric Cancer. Ann. Surg. 2012, 256, 1014–1022.
- Li, W.; Wang, D.; Sun, X.; Zhang, Y.; Wang, L.; Suo, J. ADAM17 promotes lymph node metastasis in gastric cancer via activation of the Notch and Wnt signaling pathways. Int. J. Mol. Med. 2019, 43, 914–926.
- Yang, R.; Sun, L.; Li, C.-F.; Wang, Y.-H.; Yao, J.; Li, H.; Yan, M.; Chang, W.-C.; Hsu, J.-M.; Cha, J.-H.; et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat. Commun. 2021, 12, 832.




