
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francisco Cruz-Sosa | + 4383 word(s) | 4383 | 2021-12-23 09:40:39 | | | |
| 2 | Conner Chen | Meta information modification | 4383 | 2021-12-29 01:38:06 | | |
Video Upload Options
The large-scale production of plant-derived secondary metabolites (PDSM) in bioreactors to meet the increasing demand for bioactive compounds for the treatment and prevention of degenerative diseases is nowadays considered an engineering challenge. Plant cell culture (PCC) is nowadays recognized as a promising, renewable, sustainable, and environmentally friendly alternative to obtain PDSM out of wild plants. PCC accounts for the virtues of whole-plant cultivation systems and offers significant advantages, such as controlled manufacture due to standardized environmental conditions, i.e., it is not seasonal dependent, makes use of low amounts of water, and pesticides and herbicides are not required, achieving better quality in the desired product.
1. Types of Cell Cultures
| Compound | Plant Species | Biological Activity/ Pharmaceutical Use |
Extraction Yield | Type of Culture | Ref. | |
|---|---|---|---|---|---|---|
| Mother Plant | In Vitro Cell Culture | |||||
| Shikonin | Lithospermum erythrorhizon Alkanna tinctoria Tausch |
Anticancer, antibacterial, anti-inflammatory, hepatic steatosis attenuator, antitumor, and antioxidants | 10−20 mg/g | 150−200 mg/g | CSC | [10][11][12][13][14] |
| Echium plantagineum L. | 36.25 mg/L | HRC | [15] | |||
| Anthraquinones | Morinda citrifolia Rubia cordifolia Senna obtusifolia |
Antimicrobial, antifungal, hypotensive, analgesic, antimalarial, gastroprotective, antioxidant, hepatoprotective and antileukemic, and mutagenic functions | 3 mg/g | 100–200 mg/g | HRC CCC CSC |
[16][17][18][19] |
| Rosmarinic acid | Ocimum basilicum | Antioxidant, anti-inflammatory, antiviral activities | 8.78–9.4 mg/g | 12.32–21.28 mg/g | CSC | [20][21] |
| Origanum vulgare | 23.53 mg/g | 31.25 mg/g | CSC | [22][23] | ||
| Satureja khuzistanica | 12 mg/g | 38 mg/g | CSC | [24][25] | ||
| Coleus blumei | 30 mg/g | 270 mg/g | CSC | [26] | ||
| Salvia officinalis | 30 mg/g | 360 mg/g | CSC | |||
| Berberine | Thalictrum minus | Effects antitumor, anticancer, lower blood lipid, lower blood glucose, anti-osteoporosis, anti-osteoarthritis, antibiotic, and anti-inflammatory | 0.1 mg/g | 0.8 mg/mL | CSC | [27][28][29][30][31][32] |
| Coptis japonica | 20–40 mg/g | 132 mg/g | CSC | |||
| Coscinium fenestratum | 1 mg/g | 178 mg/g | CCC | |||
| Ginsenosides | Panax ginseng | Antitumor, immunological, anti-inflammation, anticancer, antidiabetic, and cardiovascular-protective | 0.015–8 mg/g | 36.4–80 mg/g | HRC | [9][33][34] |
| 3.4–28.9 mg/g | CSC | |||||
| 15.1–105.6 mg/g | ARC | |||||
| Panax japonicus | 20–50 mg/g | CSC | ||||
| Panax notoginseng | 60 mg/g | CCC | ||||
| 71.94 mg/g | ARC | |||||
| 40 mg/g | CSC | |||||
| Diosgenin | Dioscorea deltoidea | Anticancer, antidiabetic, anticoagulant, antithrombosis, anti-inflammatory, antiviral, anti-ageing | 0.4−3 mg/g | 72 mg/g | CSC | [35] |
| 3.5–16 mg/g | CCC | |||||
| Dioscorea bulbifera | 12 mg/g | CCC | ||||
| Helicteres isora L. | 1–5 mg/g | 8.64 mg/L | CSC | [36] | ||
| 23 mg/g | CCC | [37] | ||||
| Ajmalicine | Catharanthus roseus | Antihypertensive, obstructive circulatory diseases treatment | 3 mg/g | 63 mg/L | CCC | [38] |
| 10 mg/g | CSC | [39][40] | ||||
| 34 mg/L | HRC | |||||
| Paclitaxel | Taxus chinensis | Anticancer | 0.02 mg/g | 1.5 mg/g | CSC | [41] |
| Podophyllotoxin | Linum narbonense | Vigorous antimitotic and antiviral activities and anticancer | 0.5 mg/g | 1.57 mg/g | CCC | [42] |
| Juniperus chinensi | 0.025 mg/g | 189.91 mg/g | CSC | |||
| Linum flavum | 1.6 mg/g | 2 mg/g | CSC | |||
| Artemisinin | Artemisia annua L. | Treat multi-drug-resistant strains of falciparum malaria | 1–15 mg/g | 9.33–110.2 mg/L | CSC | [43][44] |
| Phenolic Acids | Verbena officinalis | Antimicrobial, secretolytic, expectorant, and diuretic agent | 136.59 mg/g | 126.55 mg/g | CCC | [45] |
| (rosmarinic, chlorogenic, and ferulic acid) | 189.91 mg/g | CSC | ||||
| Resveratrol | Vitis vinifera L. | Reduced coronary heart disease mortality rates and atherosclerosis, inhibiting low-density lipoprotein oxidation, and carcinogenesis | NR | 277.89 µg/g | CSC | [46] |
2. Plant Cell Suspension Culture
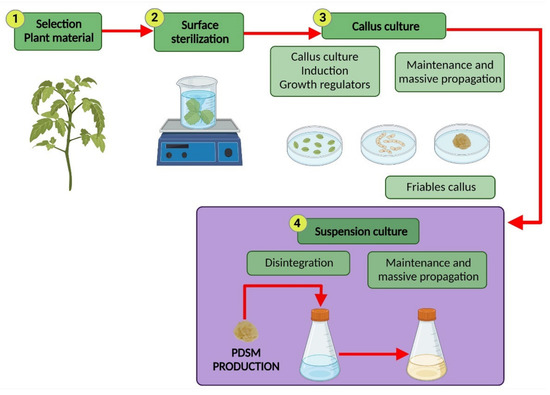
3. Commercial Production of PDSM from CSC
| Product | Species | Pharmaceutical Use | Manufacturer, Tradename, and Scale of Production |
Type of Culture | Reference |
|---|---|---|---|---|---|
| Rosmarinic acid | Coleus blumei | Anti-inflammatory | ANattermann & Cie. Gmbh, www.sanofi.de (accessed on 30 October 2021) |
CSC | [62] |
| Echinacea polysaccharides | Echinacea purpurea | Immunostimulant, anti-inflammatory | Diversa, 75,000 L bioreactor | CSC | [59][63] |
| Berberines | Thalictrum minun | Anticancer; antibiotic; anti-inflammatory | Mitsui Chemicals, Inc., (75,000 Lbr) | CSC | [64] |
| Coptis japonica | https://www.mitsuichemicals.com/ (accessed on 30 October 2021) | CSC | |||
| Podophyllotoxin | Podophyllum spp. | Anticancer | Nippon Oil Company, Ltd. | CSC | [65] |
| https://www.freepatentsonline.com/5336605.html (accessed on 30 October 2021) | OC | [66] | |||
| Docetaxel | Taxus baccata | Ovarian cancer treatment | Phyton Biotech, Inc., Taxotere (150 kg/year) | CSC | [67][68] |
| https://phytonbiotech.com/ (accessed on 30 October 2021) | |||||
| Paclitaxel | Taxus spp. | Anticancer: FDA approved for the treatment of ovarian, breast, and lung cancers | Phyton Biotech, Inc., Taxol ® (1000 kg/year) | CSC | [69] |
| https://phytonbiotech.com/ (accessed on 30 October 2021) | |||||
| Samyang Genex Corporation., Genexol (32,000 Lbr) https://samyangbiopharm.com/eng/ProductIntroduce/injection01 (accessed on 30 October 2021) | CSC | [70] | |||
| [71] | |||||
| Scopolamine | Duboisia spp. | Anticholinergic; antimuscarinic; motion sickness, nausea, and intestinal cramping | Sumitomo Chemical Co., Ltd., Tokyo, Japan (50–20,000 Lbr) https://www.sumitomo-chem.co.jp/pharma-chem/ (accessed on 30 October 2021) |
HRC | [72][73] |
| Shikonin | Lithospermum erythrorhizon | Anti-HIV, antitumor, anti-inflammatory | Xi’an NEO Biotech, Shikonin 95% | CSC | [59] |
| http://www.extractneo.com/about (accessed on 30 October 2021) |
4. Typical Bioreactor Configurations
| Bioreactor Configuration | Schematic Diagram * | Description | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Bubble column (BC) |
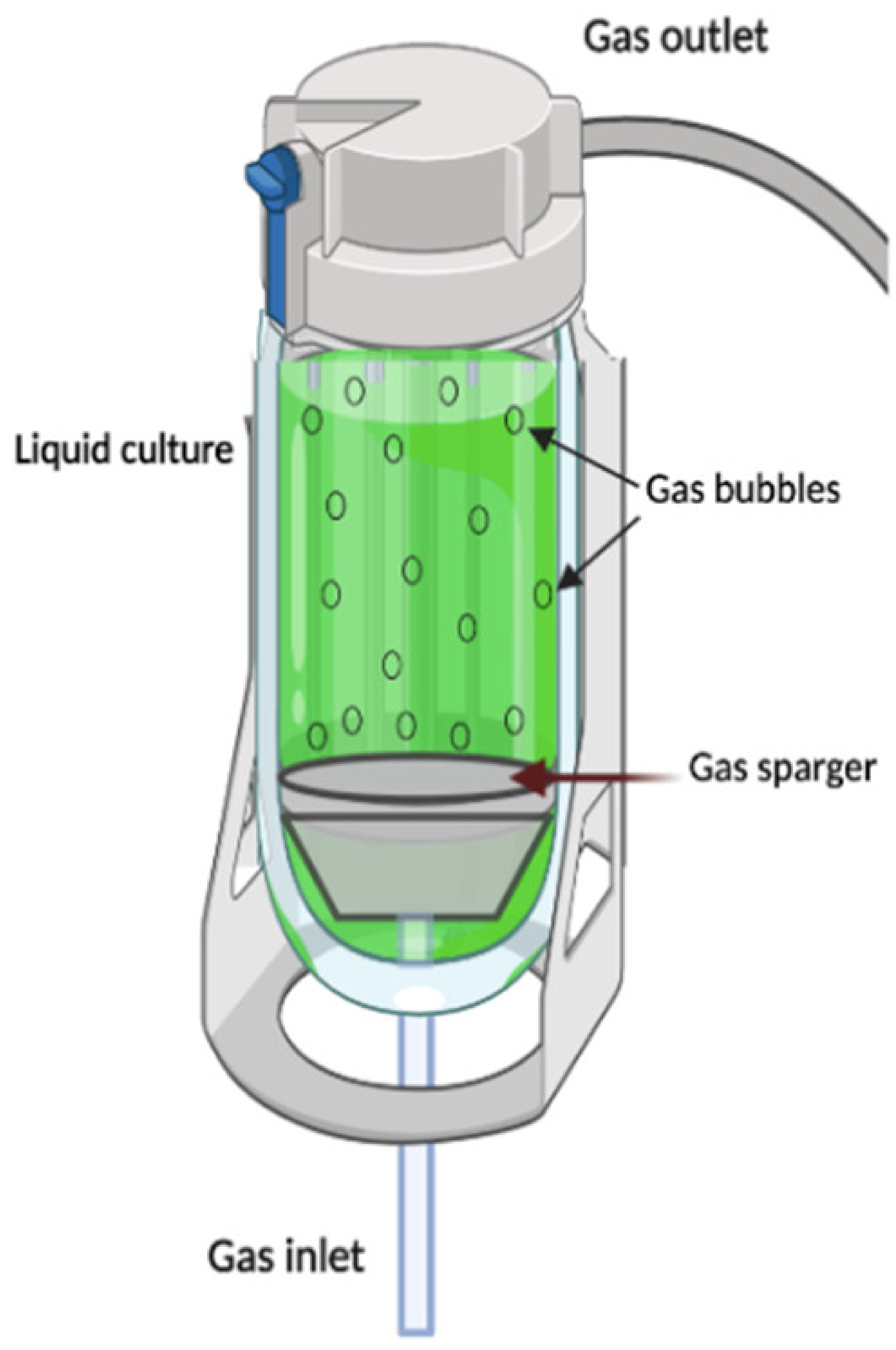 |
It is classified in the pneumatic-type bioreactor. They are constructed in cylindric columns where gas injection represents the only energy entrance to the system. BC bioreactors operate under constant bubbling where gas flows from the bottom to the top through nozzles, perforated plates, or spray rings, allowing not only the aeration process, but also helping the mixing and circulation of the fluid, without the need to install mechanical accessories. | Simple structure as no mechanical force is required to shake. Easier maintenance and reduces the risk of contamination due to the lack of mobile parts. Reduced effect of the shear stress. |
High foam formation under high gas flow rates. Poor oxygen transfer capabilities. Poor fluid mixing in highly viscous fluids. High levels of foaming under high-aeration conditions |
[58][49][75] |
| Airlift (ALB) | 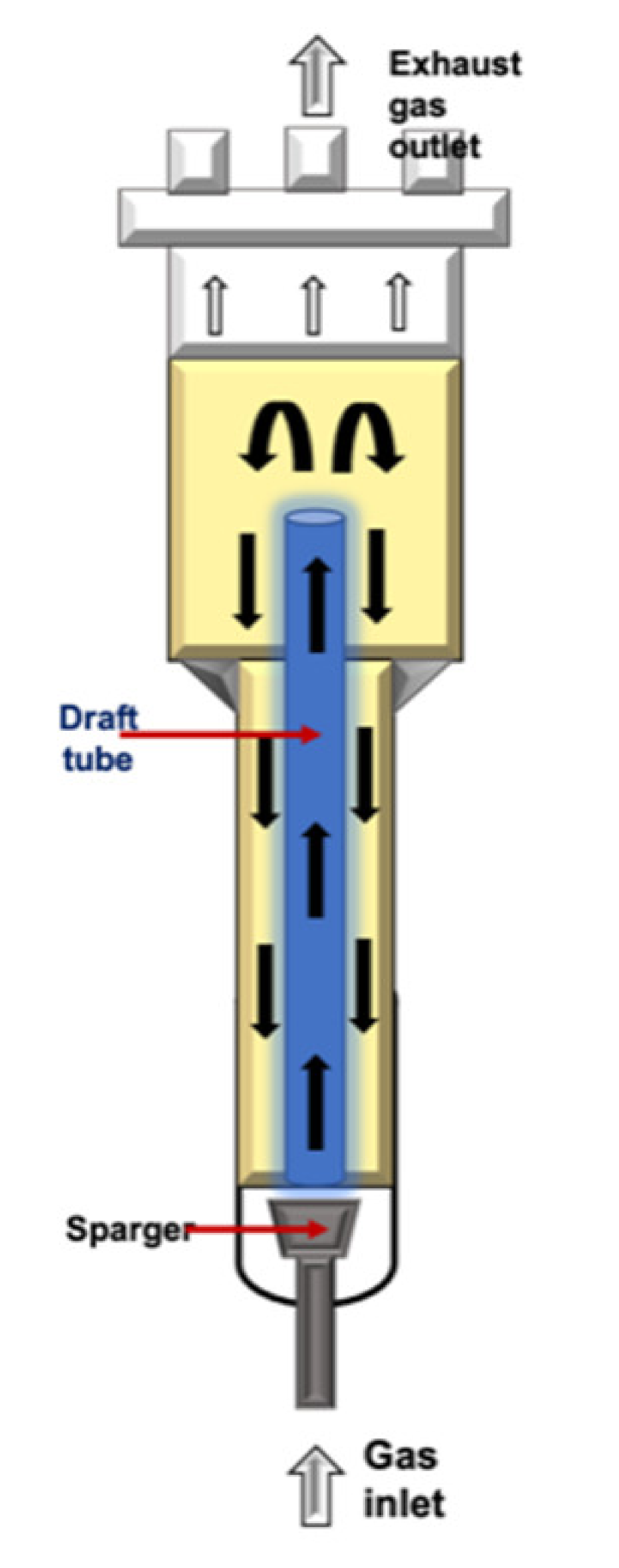 |
It is classified in the pneumatic-type bioreactor. This configuration is considered reasonably like STR, excepting for the impeller. They are tower reactors where fluid broth is mixed with a gas stream, which is compressed and injected at the bottom of the discharge pipe. The gas–fluid mix allows the creation ofdifferences in density and upward displacement. It is more suitable for hairy root and somatic embryo cultures. | Easy maintenance and reduces the risk of contamination due to the absence of mobile parts. Reduced effect of the shear stress. Higher oxygen transfer than that in BC. The energy required is provided by the compressed gas. |
High levels of foam formation under high gas flow rates. Poor fluid mixing in highly viscous fluids. Relatively poor oxygen transfer capabilities. |
[58][76][77][78] |
| Stirred tank bioreactor (STB) |  |
It is grouped in the mechanically agitated bioreactor. This bioreactor consists in a mixer (turbine or propeller) installed within the tank reactor and may be equipped with gassing inlet stream. It can operate in batch, semi-continuous, or continuous mode [76][79]. | Efficient fluid mixing systems. High oxygen mass transfer capability. Convenient for high-viscous fluids. Comply with Good Manufacturing Practices. Easy scale-up. Highly adaptable to production scale and products. Impeller alternative. |
High energy cost owing to mechanical agitation. Contamination risk with mechanical seal. Some cells and metabolites are susceptible to shearing generated by the impeller and bursting gas bubbles. Depending on the operation mode, this configuration can represent high costs of maintenance, cleaning, and startup. |
[49][76][79][80] |
Engineering Aspects in the Plant Cell Suspension Culture
| Species | Compounds | Operation Variables Evaluated | Biomass Production |
PDSM Production |
Ref. | ||
|---|---|---|---|---|---|---|---|
| In Shake Flask | In Bioreactor |
In Shake Flask | In Bioreactor | ||||
| Scrophularia striata | Phenylethanoid glycosides | 50 mL SCC in 100 mL flask 110 rpm 25 °C |
5.0 L SCC in STR 10 L Fg: 0.5–1.0 L/min 110−170 rpm 25 ± 1 °C Darkness |
14.16 g/L | 15.64 g/L | The acteoside content in CSC in the bioreactor was about threefold higher than that in the shake flask | [81] |
| Buddleja cordata | Verbascoside, linarin and hydroxycinnamic acids |
50 mL SCC in 250 mL flasks 110 rpm 26 ± 2 °C |
STR 2 L Fg: 1 vvm (ring diffuser Rushton impeller 400 rpm 26 ± 2 °C 16/8 h light to dark photoperiod |
11.8 g/L | 13.62 g/L | The content of phenolics was twofold higher in STR. | [82][83] |
| Rubia tinctorum |
Anthraquinone | 25 mL SCC in 250 mL flasks 100 rpm 25 ± 2 °C 16/8 h photoperiod (140 µmol m−2 s−1) |
1.0 L SCC in STR 2 L Fg: 1 vvm Turbine impeller 450 rpm 25 ± 2 °C 16/8 h photoperiod (140 µmol m−2 s−1) |
330 g/L | 220 g/L | Anthroquinone production was 2.5 times higher in STR | [84] |
| Arnebia sp. | Shikonin | 25 mL CSC in 250 mL flasks | Air-lift bioreactor | 1249.2 g/L | 480 g/L | The shikonin content was 2.6 times higher in the bioreactor than in the flask. Production remained without significant differences in both bioreactors | [85] |
| 100 rpm | 2 L working volume | ||||||
| 25 ± 2 °C | 25 ± 2 °C | ||||||
| Continuous light | Fg: 2 L/min (sparger ring) | ||||||
| (70 µmol/m2 s 1) | |||||||
| STR 2 L | 1249.2 g/L | 450 g/L | |||||
| Six-blade turbine impeller 100 rpm | |||||||
| Fg: 2 L/min | |||||||
| 25 ± 2 °C | |||||||
| Ocinum basilicum | Rosmarinic acid | 100 rpm | 7 L CSC in STR 10 L | Biomass was 8.4 times higher in bioreactor than in flask | Production increased 1.66 times in bioreactor | [21] | |
| 25 ± 2 °C | Marine impeller 100 rpm | ||||||
| Fg: 25 L/min | |||||||
| Satureja khuzistanica | Rosmarinic acid | 200 mL CSC in 1 L flask | 1 L CSC in culture bags 2 L | 13.6 g/L | 18.7 g/L | Production increased 2.5 times in bioreactor | [86] |
| 110 rpm | Batch mode | ||||||
| 25 °C | 20–30 rpm | ||||||
| 25 °C | |||||||
| Fg: 0.1 vvm | |||||||
| Darkness | |||||||
| Vitis labrusca L. | Resveratrol | 100 mL CSC in 300 mL flasks | STR 5 L | NR | ≈35 g DW | Production increased 1.15 times in bioreactor | [87] |
| 110 rpm | Marine impeller 110 rpm | ||||||
| 23 °C | Fg: 0.15 vvm | ||||||
| Darkness | |||||||
| Santalum album L. | Squalene | 100 mL CSC in 250 L flask | Airlift bioreactor 7 L | 1.05 mg/g | 1.25 mg/g | Production increased 1.71 times in bioreactor in four weeks of culture | [88] |
| 90 rpm | Batch mode | ||||||
| 28 °C | 70–80 rpm | ||||||
| Fg: 4 L/min | |||||||
| 28 ± 2 ° C | |||||||
| Mathematical Equation | Conventional Name |
|---|---|
| rx=µ=µmax[Si][Si]+Kmrs=Yxsµ | Monod kinetics |
| rx=µ=µmax[Si]([Si]2/Ki)+[Si]+Kmrs=Yxsµ | Expanded Monod kinetics |
| rx=µ=µmax[Si][Si]+Km(1−[P][P]max)rs=Yxsµ | Expanded Monod kinetics |
| rx=µ=µmax(1−exp(−[Si]/Km))rs=Yxsµ | Monod’s teacher Tessier kinetics. |
| rx=µ=µmax[Si][Si]+KSXrs=Yxsµ | Contois kinetics. |
| rx=µ=µmax(1−XKS)rs=Yxsµ | Logistic kinetics. |
| dθxdt=−kin(θx−θss)mr=rs=θxk[Si][Si]+Km | Cell deactivation kinetics |
- (1)
-
It defines an establishment of the relationship between responses (yield, cell viability, oxygen concentration, etc.) and control operating conditions (temperature, pressure, initial concentration, power input, agitation rate, etc.).
- (2)
-
It predicts the effect of control operating condition on responses.
- (3)
-
It gives inferences on the significance of the operating conditions on the performance of the reactor.
- (4)
-
It allows the determination of the operating window where the bioreactor meets its best performance.
References
- Kwon, Y.-W.; Lee, S.-H.; Kim, A.-R.; Kim, B.J.; Park, W.-S.; Hur, J.; Jang, H.; Yang, H.-M.; Cho, H.-J.; Kim, H.-S. Plant callus-derived shikimic acid regenerates human skin through converting human dermal fibroblasts into multipotent skin-derived precursor cells. Stem Cell Res. Ther. 2021, 12, 346.
- Furusaki, S.; Takeda, T. Bioreactors for plant cell culture. Compr. Biotechnol. 2017, 519–530.
- Gonçalves, S.; Romano, A. Production of plant secondary metabolites by using biotechnological tools. In Secondary Metabolites, Sources and Applications; IntechOpen: London, UK, 2018.
- Isah, T.; Umar, S.; Mujib, A.; Sharma, M.P.; Rajasekharan, P.E.; Zafar, N.; Frukh, A. Secondary metabolism of pharmaceuticals in the plant in vitro cultures: Strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue Organ Cult. 2018, 132, 239–265.
- Prasad, G.D.; Sudina, B.; Janardan, L.; Rajani, S.; Rosario, G.-G.M. Establishment of regenerative callus, cell suspension system, and molecular characterization of Taxus wallichiana Zucc. for the in vitro production of Taxol. J. Appl. Pharm. Sci. 2020, 11, 22–34.
- Sharma, K.; Zafar, R. Optimization of methyl jasmonate and β-cyclodextrin for enhanced production of taraxerol and taraxasterol in (Taraxacum officinale Weber) cultures. Plant Physiol. Biochem. 2016, 103, 24–30.
- Chastang, T.; Pozzobon, V.; Taidi, B.; Courot, E.; Clément, C.; Pareau, D. Resveratrol production by grapevine cells in fed-batch bioreactor: Experiments and modelling. Biochem. Eng. J. 2018, 131, 9–16.
- Jeong, Y.J.; Park, S.H.; Park, S.-C.; Kim, S.; Kim, T.H.; Lee, J.; Kim, S.W.; Ryu, Y.B.; Jeong, J.C.; Kim, C.Y. Induced extracellular production of stilbenes in grapevine cell culture medium by elicitation with methyl jasmonate and stevioside. Bioresour. Bioprocess. 2020, 7, 40643.
- Le, K.-C.; Jeong, C.-S.; Lee, H.; Paek, K.-Y.; Park, S.-Y. Ginsenoside accumulation profiles in long- and short-term cell suspension and adventitious root cultures in Panax ginseng. Hortic. Environ. Biotechnol. 2018, 60, 125–134.
- Malik, S.; Cusido, R.M.; Mirjalili, M.H.; Moyano, E.; Palazon, J.; Bonfill, M. Production of the anticancer drug taxol in Taxus baccata suspension cultures: A review. Process. Biochem. 2011, 46, 23–34.
- Sahakyan, N.; Petrosyan, M.; Trchounian, A. The activity of Alkanna species in vitro culture and intact plant extracts against antibiotic resistant bacteria. Curr. Pharm. Des. 2019, 25, 1861–1865.
- Gwon, S.Y.; Ahn, J.; Jung, C.H.; Moon, B.; Ha, T.-Y. Shikonin attenuates hepatic steatosis by enhancing beta oxidation and energy expenditure via AMPK activation. Nutrients 2020, 12, 1133.
- Jeziorek, M.; Damianakos, H.; Kawiak, A.; Laudy, A.E.; Zakrzewska, K.; Sykłowska-Baranek, K.; Chinou, I.; Pietrosiuk, A. Bioactive rinderol and cynoglosol isolated from Cynoglossum columnae Ten. in vitro root culture. Ind. Crop. Prod. 2019, 137, 446–452.
- Rat, A.; Naranjo, H.D.; Krigas, N.; Grigoriadou, K.; Maloupa, E.; Alonso, A.V.; Schneider, C.; Papageorgiou, V.P.; Assimopoulou, A.N.; Tsafantakis, N.; et al. Endophytic bacteria from the roots of the medicinal plant Alkanna tinctoria Tausch (Boraginaceae): Exploration of plant growth promoting properties and potential role in the production of plant secondary metabolites. Front. Microbiol. 2021, 12, 113.
- Fu, J.-Y.; Zhao, H.; Bao, J.-X.; Wen, Z.-L.; Fang, R.-J.; Fazal, A.; Yang, M.-K.; Liu, B.; Yin, T.-M.; Pang, Y.-J.; et al. Establishment of the hairy root culture of Echium plantagineum L. and its shikonin production. 3 Biotech 2020, 10, 429.
- Baque, A.; Shiragi, H.K.; Moh, S.-H.; Lee, E.-J.; Paek, K.-Y. Production of biomass and bioactive compounds by adventitious root suspension cultures of Morinda citrifolia (L.) in a liquid-phase airlift balloon-type bioreactor. Vitr. Cell. Dev. Biol. Anim. 2013, 49, 737–749.
- Veremeichik, G.; Bulgakov, V.; Shkryl, Y.; Silantieva, S.; Makhazen, D.; Tchernoded, G.; Mischenko, N.; Fedoreyev, S.; Vasileva, E. Activation of anthraquinone biosynthesis in long-cultured callus culture of Rubia cordifolia transformed with the rolA plant oncogene. J. Biotechnol. 2019, 306, 38–46.
- Mariadoss, A.; Satdive, R.; Fulzele, D.P.; Ramamoorthy, S.; Zayed, H.; Younes, S.; Rajasekaran, C. Enhanced production of anthraquinones by gamma-irradiated cell cultures of Rubia cordifolia in a bioreactor. Ind. Crop. Prod. 2020, 145, 111987.
- Kowalczyk, T.; Sitarek, P.; Toma, M.; Rijo, P.; Domínguez-Martíne, E.; Falcó, I.; Sánchez, G.; Śliwiński, T. Enhanced accumulation of betulinic acid in transgenic hairy roots of Senna obtusifolia growing in the Sprinkle Bioreactor and evaluation of their biological properties in various biological models. Chem. Biodivers. 2021, 18, e2100455.
- Açıkgöz, M.A. Effects of sorbitol on the production of phenolic compounds and terpenoids in the cell suspension cultures of Ocimum basilicum L. Biologia 2021, 76, 395–409.
- Pandey, P.; Singh, S.; Banerjee, S. Ocimum basilicum suspension culture as resource for bioactive triterpenoids: Yield enrichment by elicitation and bioreactor cultivation. Plant Cell Tissue Organ Cult. 2019, 137, 65–75.
- Li, Y.-P.; Tang, D.-B.; Wang, X.-Q.; Wang, M.; Zhang, Q.-F.; Liu, Y.; Shen, B.-Y.; Chen, J.-G.; Yin, Z.-P. Development of Origanum vulgare cell suspension culture to produce polyphenols and the stimulation effect of salicylic acid elicitation and phenylalanine feeding. Biotechnol. Bioprocess Eng. 2021, 26, 456–467.
- Gonçalves, S.; Moreira, E.; Grosso, C.; Andrade, P.B.; Valentão, P.; Romano, A. Phenolic profile, antioxidant activity and enzyme inhibitory activities of extracts from aromatic plants used in Mediterranean diet. J. Food Sci. Technol. 2017, 54, 219–227.
- Sahraroo, A.; Mirjalili, M.H.; Corchete, P.; Babalar, M.; Moghadam, M.R.F. Establishment and characterization of a Satureja khuzistanica Jamzad (Lamiaceae) cell suspension culture: A new in vitro source of rosmarinic acid. Cytotechnology 2016, 68, 1415–1424.
- Sahraroo, A.; Mirjalili, M.H.; Babalar, M.; Zarei, A. Enhancement of rosmarinic acid production by Satureja khuzistanica cell suspensions: Effects of phenylalanine and sucrose. SABRAO J. Breed. Genet. 2018, 50, 25–35.
- Smetanska, I. Sustainable Production of Polyphenols and Antioxidants by Plant In Vitro Cultures; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9783319545998.
- Och, A.; Podgórski, R.; Nowak, R. Biological activity of berberine—A summary update. Toxins 2020, 12, 713.
- Khan, T.; Krupadanam, D.; Anwar, S.Y. The role of phytohormone on the production of berberine in the calli cultures of an endangered medicinal plant, turmeric (Coscinium fenestratum L.). Afr. J. Biotechnol. 2008, 7, 3244–3246.
- Yamada, Y.; Yoshimoto, T.; Yoshida, S.T.; Sato, F. Characterization of the promoter region of biosynthetic enzyme genes involved in Berberine Biosynthesis in Coptis japonica. Front. Plant Sci. 2016, 7, 1352.
- Cheung, C.K.-L.; Leksawasdi, N.; Doran, P.M. Bioreactor scale-down studies of suspended plant cell cultures. AIChE J. 2018, 64, 4281–4288.
- Tabata, M. Transport and secretion of natural products in plant cell cultures. Planta Med. 1991, 57, S21–S26.
- Sato, F.; Yamada, Y. High berberine-producing cultures of coptis japonica cells. Phytochemistry 1984, 23, 281–285.
- Hou, M.; Wang, R.; Zhao, S.; Wang, Z. Ginsenosides in Panax genus and their biosynthesis. Acta Pharm. Sin. B 2021, 11, 1813–1834.
- Adil, M.; Jeong, B.R. In vitro cultivation of Panax ginseng C.A. Meyer. Ind. Crop. Prod. 2018, 122, 239–251.
- Nazir, R.; Kumar, V.; Gupta, S.; Dwivedi, P.; Pandey, D.K.; Dey, A. Biotechnological strategies for the sustainable production of diosgenin from Dioscorea spp. Appl. Microbiol. Biotechnol. 2021, 105, 569–585.
- Shaikh, S.; Shriram, V.; Khare, T.; Kumar, V. Biotic elicitors enhance diosgenin production in Helicteres isora L. suspension cultures via up-regulation of CAS and HMGR genes. Physiol. Mol. Biol. Plants 2020, 26, 593–604.
- Deshpande, H.A.; Bhalsing, S.R. Isolation and characterization of diosgenin from in vitro cultured tissues of Helicteres isora L. Physiol. Mol. Biol. Plants 2013, 20, 89–94.
- Das, A.; Sarkar, S.; Bhattacharyya, S.; Gantait, S. Biotechnological advancements in Catharanthus roseus (L.) G. Don. Appl. Microbiol. Biotechnol. 2020, 104, 4811–4835.
- Thakore, D.; Srivastava, A.; Sinha, A.K. Mass production of Ajmalicine by bioreactor cultivation of hairy roots of Catharanthus roseus. Biochem. Eng. J. 2017, 119, 84–91.
- Alamgir, A.N.M. Cultivation of herbal drugs, biotechnology, and in vitro production of secondary metabolites, high-value medicinal plants, herbal wealth, and herbal trade. In Therapeutic Use of Medicinal Plants and Their Extracts: Volume 1; Springer: Berlin/Heidelberg, Germany, 2017; pp. 379–452. ISBN 9783319638621.
- Changxing, L.; Galani, S.; Hassan, F.-U.; Rashid, Z.; Naveed, M.; Fang, D.; Ashraf, A.; Qi, W.; Arif, A.; Saeed, M.; et al. Biotechnological approaches to the production of plant-derived promising anticancer agents: An update and overview. Biomed. Pharmacother. 2020, 132, 110918.
- Kumar, P.; Sharma, P.; Kumar, V.; Kumar, A. Plant resources: In vitro production, challenges and prospects of secondary Metabolites from medicinal plants. Ind. Biotechnol. 2019, 2019, 89–104.
- Salehi, M.; Karimzadeh, G.; Naghavi, M.R. Synergistic effect of coronatine and sorbitol on artemisinin production in cell suspension culture of Artemisia annua L. cv. Anamed. Plant Cell Tissue Organ Cult. 2019, 137, 587–597.
- Kayani, W.K.; Kiani, B.H.; Dilshad, E.; Mirza, B. Biotechnological approaches for artemisinin production in Artemisia. World J. Microbiol. Biotechnol. 2018, 34, 54.
- Kubica, P.; Szopa, A.; Kokotkiewicz, A.; Miceli, N.; Taviano, M.; Maugeri, A.; Cirmi, S.; Synowiec, A.; Gniewosz, M.; Elansary, H.; et al. Production of Verbascoside, Isoverbascoside and Phenolic acids in callus, suspension, and bioreactor cultures of Verbena officinalis and biological properties of biomass extracts. Molecules 2020, 25, 5609.
- Süntar, I.; Çetinkaya, S.; Haydaroğlu, Ü.S.; Habtemariam, S. Bioproduction process of natural products and biopharmaceuticals: Biotechnological aspects. Biotechnol. Adv. 2021, 50, 107768.
- Yue, W.; Ming, Q.-L.; Lin, B.; Rahman, K.; Zheng, C.-J.; Han, T.; Qin, L.-P. Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232.
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant cell culture technology in the cosmetics and food industries: Current state and future trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675.
- Valdiani, A.; Hansen, O.K.; Nielsen, U.B.; Johannsen, V.K.; Shariat, M.; Georgiev, M.I.; Omidvar, V.; Ebrahimi, M.; Dinanai, E.T.; Abiri, R. Bioreactor-based advances in plant tissue and cell culture: Challenges and prospects. Crit. Rev. Biotechnol. 2018, 39, 20–34.
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.; Jang, M.O.; Jin, Y.-W.; Lee, E.-K.; Loake, G.J. Plant cell culture strategies for the production of natural products. BMB Rep. 2016, 49, 149–158.
- Bhaskar, R.; Xavier, L.S.E.; Udayakumaran, G.; Kumar, D.S.; Venkatesh, R.; Nagella, P. Biotic elicitors: A boon for the in-vitro production of plant secondary metabolites. Plant Cell Tissue Organ Cult. 2021, 147, 1–18.
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12.
- Narayani, M.; Srivastava, S. Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem. Rev. 2017, 16, 1227–1252.
- Nabi, N.; Singh, S.; Saffeullah, P. Responses of in vitro cell cultures to elicitation: Regulatory role of jasmonic acid and methyl jasmonate: A review. Vitr. Cell. Dev. Biol. Anim. 2021, 57, 341–355.
- Khan, T.; Khan, T.; Hano, C.; Abbasi, B.H. Effects of chitosan and salicylic acid on the production of pharmacologically attractive secondary metabolites in callus cultures of Fagonia indica. Ind. Crop. Prod. 2019, 129, 525–535.
- Kehie, M.; Kumaria, S.; Tandon, P. Biotechnological enhancement of capsaicin biosynthesis in cell suspension cultures of Naga King Chili (Capsicum chinense Jacq.). Bioprocess Biosyst. Eng. 2016, 39, 205–210.
- Schenke, D.; Utami, H.P.; Zhou, Z.; Gallegos, M.-T.; Cai, D. Suppression of UV-B stress induced flavonoids by biotic stress: Is there reciprocal crosstalk? Plant Physiol. Biochem. 2019, 134, 53–63.
- Werner, S.; Maschke, R.W.; Eibl, D.; Eibl, R. Bioreactor technology for sustainable production of plant cell-derived products. In Bioprocessing of Plant In Vitro Systems; Springer: Cham, Switzerland, 2018; pp. 413–432.
- Lange, B.M. Commercial-scale tissue culture for the production of plant natural products: Successes, failures and outlook. In Biotechnology of Natural Products; Springer: Cham, Switzerland, 2018; pp. 189–218.
- Marketwatch. Available online: https://www.marketwatch.com/press-release/paclitaxel-market-size-in-2021-87-cagr-with-top-countries-data-competition-strategies-share-industry-analysis-by-top-manufactures-growth-insights-and-forecasts-to-2026-2021-08-06 (accessed on 8 December 2021).
- Frense, D. Taxanes: Perspectives for biotechnological production. Appl. Microbiol. Biotechnol. 2007, 73, 1233–1240.
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18.
- Wagner, H.; Stuppner, H.; Schäfer, W.; Zenk, M. Immunologically active polysaccharides of Echinacea purpurea cell cultures. Phytochemistry 1988, 27, 119–126.
- DiCosmo, F.; Misawa, M. Plant cell and tissue culture: Alternatives for metabolite production. Biotechnol. Adv. 1995, 13, 425–453.
- Giri, A.; Narasu, M.L. Production of podophyllotoxin from Podophyllum hexandrum: A potential natural product for clinically useful anticancer drugs. Cytotechnology 2000, 34, 17–26.
- Sasheva, P.; Ionkova, I. Small Cells for Big Ideas: The Cytotoxic Podophyllotoxin and the Long Journey in Discovering Its Biosynthetic Pathway. In Biotechnology and Production of Anti-Cancer Compounds; Federal University of Maranhao: Sao Luis, Brazil, 2017.
- Rao, K.; Chodisetti, B.; Gandi, S.; Giri, A.; Kishor, P.B.K. Cadmium chloride elicitation of Abutilon indicum cell suspension cultures for enhanced stigmasterol production. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2021, 155, 1–6.
- Ojha, T.; Hu, Q.; Colombo, C.; Wit, J.; van Geijn, M.; van Steenbergen, M.J.; Bagheri, M.; Königs-Werner, H.; Buhl, E.M.; Bansal, R.; et al. Lyophilization stabilizes clinical-stage core-crosslinked polymeric micelles to overcome cold chain supply challenges. Biotechnol. J. 2021, 16, 2000212.
- McElroy, C.; Jennewein, S. Taxol® biosynthesis and production: From forests to fermenters. In Biotechnology of Natural Products; Springer: Berlin/Heidelberg, Germany, 2017; pp. 145–185.
- Chattopadhyay, S.; Farkya, S.; Srivastava, A.; Bisaria, V.S. Bioprocess considerations for production of secondary metabolites by plant cell suspension cultures. Biotechnol. Bioprocess Eng. 2002, 7, 138–149.
- Lee, S.-W.; Kim, Y.-M.; Cho, C.H.; Kim, Y.T.; Kim, S.M.; Hur, S.Y.; Kim, J.-H.; Kim, B.-G.; Kim, S.-C.; Ryu, H.-S.; et al. An open-label, randomized, parallel, phase ii trial to evaluate the efficacy and safety of a cremophor-free polymeric micelle formulation of paclitaxel as first-line treatment for ovarian cancer: A Korean gynecologic oncology group study (KGOG-3021). Cancer Res. Treat. 2018, 50, 195–203.
- Muranaka, T.; Ohkawa, H.; Yamada, Y. Continuous production of scopolamine by a culture of Duboisia leichhardtii hairy root clone in a bioreactor system. Appl. Microbiol. Biotechnol. 1993, 40, 219–223.
- D’Amelia, V.; Docimo, T.; Crocoll, C.; Rigano, M. Specialized metabolites and valuable molecules in crop and medicinal plants: The evolution of their use and strategies for their production. Genes 2021, 12, 936.
- Singh, J.; Kaushik, N.; Biswas, S. Bioreactors—Technology & design analysis. Scitech J. 2014, 1, 28–36.
- Esperança, M.N.; Mendes, C.E.; Rodriguez, G.Y.; Cerri, M.O.; Béttega, R.; Badino, A.C. Sparger design as key parameter to define shear conditions in pneumatic bioreactors. Biochem. Eng. J. 2020, 157, 107529.
- Barragán, L.P.; Figueroa, J.; Durán, L.R.; González, C.A.; Hennigs, C. Fermentative Production Methods; Elsevier: Amsterdam, The Netherlands, 2016; pp. 189–217.
- Zhang, T.; We, C.; Ren, Y.; Feng, C.; Wu, H. Advances in airlift reactors: Modified design and optimization of operation conditions. Rev. Chem. Eng. 2017, 33, 163–182.
- Kumar, N.; Gupta, R.; Bansal, A. Effect of surface tension on hydrodynamics and mass transfer coefficient in airlift reactors. Chem. Eng. Technol. 2020, 43, 995–1004.
- Tervasmäki, P.; Latva-Kokko, M.; Taskila, S.; Tanskanen, J. Effect of oxygen transfer on yeast growth—Growth kinetic and reactor model to estimate scale-up effects in bioreactors. Food Bioprod. Process. 2018, 111, 129–140.
- Fitzpatrick, J.J. Insights from mathematical modelling into energy requirement and process design of continuous and batch stirred tank aerobic bioreactors. ChemEngineering 2019, 3, 65.
- Ahmadi-Sakha, S.; Sharifi, M.; Niknam, V. Bioproduction of phenylethanoid glycosides by plant cell culture of Scrophularia striata Boiss.: From shake-flasks to bioreactor. Plant Cell Tissue Organ Cult. 2016, 124, 275–281.
- Estrada-Zúñiga, M.E.; Cruz-Sosa, F.; Rodriguez-Monroy, M.; Verde-Calvo, J.R.; Vernon-Carter, E.J. Phenylpropanoid production in callus and cell suspension cultures of Buddleja cordata Kunth. Plant Cell Tissue Organ Cult. 2009, 97, 39–47.
- Vazquez-Marquez, A.M.; Zepeda-Gómez, C.; Burrola-Aguilar, C.; Bernabé-Antonio, A.; Nieto-Trujillo, A.; Cruz-Sosa, F.; Rodríguez-Monroy, M.; Estrada-Zúñiga, M.E. Effect of stirring speed on the production of phenolic secondary metabolites and growth of Buddleja cordata cells cultured in mechanically agitated bioreactor. Plant Cell Tissue Organ Cult. 2019, 139, 155–166.
- Busto, V.; Rodriguez-Talou, J.; Giulietti, A.; Merchuk, J. Effect of shear stress on Anthraquinones production by Rubia tinctorum suspension cultures. Biotechnol. Prog. 2008, 24, 175–181.
- Gupta, K.; Garg, S.; Singh, J.; Kumar, M. Enhanced production of napthoquinone metabolite (shikonin) from cell suspension culture of Arnebia sp. and its up-scaling through bioreactor. 3 Biotech 2014, 4, 263–273.
- Khojasteh, A.; Mirjalili, M.H.; Palazon, J.; Eibl, R.; Cusido, R.M. Methyl jasmonate enhanced production of rosmarinic acid in cell cultures of Satureja khuzistanicain a bioreactor. Eng. Life Sci. 2016, 16, 740–749.
- Lambert, C.; Lemaire, J.; Auger, H.; Guilleret, A.; Reynaud, R.; Clément, C.; Courot, E.; Taidi, B. Optimize, modulate, and scale-up resveratrol and resveratrol dimers bioproduction in Vitis labrusca L. Cell suspension from Flasks to 20 L Bioreactor. Plants 2019, 8, 567.
- Rani, A.; Meghana, R.; Kush, A. Squalene production in the cell suspension cultures of Indian sandalwood (Santalum album L.) in shake flasks and air lift bioreactor. Plant Cell Tissue Organ Cult. 2018, 135, 155–167.
- Salehi, M.; Farhadi, S.; Moieni, A.; Safaie, N.; Ahmadi, H. Mathematical modeling of growth and paclitaxel biosynthesis in Corylus avellana cell culture responding to fungal elicitors using multilayer perceptron-genetic algorithm. Front. Plant Sci. 2020, 11, 1148.
- Maschke, R.; Geipel, K.; Bley, T. Modeling of plant in vitro cultures: Overview and estimation of biotechnological processes. Biotechnol. Bioeng. 2014, 112, 1–12.
- Villegas, A.; Arias, J.P.; Aragón, D.; Ochoa, S.; Arias, M. Structured model and parameter estimation in plant cell cultures of Thevetia peruviana. Bioprocess Biosyst. Eng. 2016, 40, 573–587.
- Chattopadhyay, S.; Bisaria, V.S.; Srivastava, A.K. Enhanced production of Podophyllotoxin by Podophyllum hexandrum using in situ cell retention bioreactor. Biotechnol. Prog. 2003, 19, 1026–1028.
- Prakash, G.; Srivastava, A.K. Modeling of azadirachtin production by Azadirachta indica and its use for feed forward optimization studies. Biochem. Eng. J. 2006, 29, 62–68.
- Amdoun, R.; Khelifi, L.; Khelifi-Slaoui, M.; Amroune, S.; Benyoussef, E.-H.; Thi, D.V.; Assaf-Ducrocq, C.; Gontier, E. Influence of minerals and elicitation on Datura stramonium L. tropane alkaloid production: Modelization of the in vitro biochemical response. Plant Sci. 2009, 177, 81–87.
- Thakore, D.; Srivastava, A.K.; Sinha, A.K. Model based fed batch cultivation and elicitation for the overproduction of ajmalicine from hairy roots of Catharanthus roseus. Biochem. Eng. J. 2015, 97, 73–80.
- Salehi, M.; Farhadi, S.; Moieni, A.; Safaie, N.; Hesami, M. A hybrid model based on general regression neural network and fruit fly optimization algorithm for forecasting and optimizing paclitaxel biosynthesis in Corylus avellana cell culture. Plant Methods 2021, 17, 13.
- Villadsen, J.; Nielsen, J.; Lidén, G. Chemicals from metabolic pathways. In Bioreaction Engineering Principles; Springer: Boston, MA, USA, 2011; pp. 7–62. ISBN 97814419968792.
- Melgarejo-Torres, R.; Castillo-Araiza, C.O.; López-Ordaz, P.; Torres-Martínez, D.; Gutiérrez-Rojas, M.; Lye, G.; Huerta-Ochoa, S. Kinetic mathematical model for ketone bioconversion using Escherichia coli TOP10 pQR239. Chem. Eng. J. 2014, 240, 1–9.
- Palmerín-Carreño, D.; Castillo-Araiza, C.; Rutiaga-Quiñones, O.; Verde-Calvo, J.; Huerta-Ochoa, S. Kinetic, oxygen mass transfer and hydrodynamic studies in a three-phase stirred tank bioreactor for the bioconversion of (+)-valencene on Yarrowia lipolytica 2.2ab. Biochem. Eng. J. 2016, 113, 37–46.
- Castillo-Araiza, C.; Palmerín-Carreño, D.; Prado-Barragán, A.; Huerta-Ochoa, S. On the conceptual design of a partitioning technology for the bioconversion of (+)-valencene to (+)-nootkatone on whole cells: Experimentation and modelling. Chem. Eng. Process. Process. Intensif. 2017, 122, 493–507.
- Liu, Y. A simple thermodynamic approach for derivation of a general Monod equation for microbial growth. Biochem. Eng. J. 2006, 31, 102–105.
- Liu, Y. Overview of some theoretical approaches for derivation of the Monod equation. Appl. Microbiol. Biotechnol. 2007, 73, 1241–1250.
- Wang, J.D.; Levin, P.A. Metabolism, cell growth and the bacterial cell cycle. Nat. Rev. Genet. 2009, 7, 822–827.
- Henson, A.M. Dynamic modeling of microbial cell populations. Curr. Opin. Biotechnol. 2003, 14, 460–467.
- Daugulis, A.J. Partitioning bioreactors. Curr. Opin. Biotechnol. 1997, 8, 169–174.
- Kalil, S.; Maugeri, F.; Rodrigues, M. Response surface analysis and simulation as a tool for bioprocess design and optimization. Process. Biochem. 2000, 35, 539–550.
- Ríos-Morales, D.; Castillo-Araiza, C.O.; Vizcarra-Mendoza, M.G. Study of the agglomeration mechanism of a natural organic solid in a bench-scale wet fluidized bed using statistical analysis and discretized population balance. Chem. Eng. Commun. 2014, 201, 23–40.
- Khuri, A.I.; Mukhopadhyay, S. Response surface methodology. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 128–149.
- Dellino, G.; Kleijnen, J.P.; Meloni, C. Robust optimization in simulation: Taguchi and response surface methodology. Int. J. Prod. Econ. 2010, 125, 52–59.
- Anderson-Cook, C.M.; Borror, C.M.; Montgomery, D.C. Response surface design evaluation and comparison. J. Stat. Plan. Inference 2009, 139, 629–641.
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley: Hoboken, NJ, USA, 2013; ISBN 9781118146927.
- Box, G. JS hunter, WG hunter. Stat. Exp. Des. Innov. Discov. 2005, 21, 303–304.
- Hanrahan, G.; Lu, K. Application of factorial and response surface methodology in modern experimental design and optimization. Crit. Rev. Anal. Chem. 2006, 36, 141–151.




