
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lara Ahmad | + 3108 word(s) | 3108 | 2021-12-17 08:40:17 | | | |
| 2 | Amina Yu | + 4 word(s) | 3112 | 2021-12-27 03:18:28 | | |
Video Upload Options
Preliminary but convergent findings suggest a role for microRNAs (miRNAs) in the generation and maintenance of chronic pain and migraine. Initial observations showed that serum levels of miR-382-5p and miR-34a-5p expression were increased in serum during the migraine attack, with miR-382-5p increasing in the interictal phase as well. By contrast, miR-30a-5p levels were lower in migraine patients compared to healthy controls. Of note, antimigraine treatments proved to be capable of influencing the expression of these miRNAs. Altogether, these observations suggest that miRNAs may represent migraine biomarkers, but several points are yet to be elucidated. A major concern is that these miRNAs are altered in a broad spectrum of painful and non-painful conditions, and thus it is not possible to consider them as truly “migraine-specific” biomarkers. These miRNAs may represent useful tools to uncover and define different phenotypes across the migraine spectrum with different treatment susceptibilities and clinical features, although further studies are needed to confirm the hypothesis. In this narrative entry , an update and a critical analysis of available data on miRNAs and migraines was provided, in order to propose possible interpretations. The main objective is to stimulate research in an area that holds promise when it comes to providing reliable biomarkers for theoretical and practical scientific advances.
1. Current evidence on miRNA expression in migraine.
| miRNA | Specimen | Population | Summary of Study Results | Reference |
|---|---|---|---|---|
| miR-382-5p | Serum | 8 migraine patients without any medication 12 migraine patients with normal medication habits 8 healthy controls |
4.1-fold increase expression during migraine attack. Higher expression during the interictal period in migraine patients vs. healthy subjects. |
Andersen et al. (2016) [1] |
| Peripheral blood mononuclear cells | 27 patients with EM 28 patients with CM–MOH |
Higher levels in CM–MOH vs. EM. Positive correlation with CGRP levels. |
Greco et al. (2020) [2] | |
| miR-34a-5p | Serum | 8 migraine patients without any medication 12 migraine patients with normal medication habits 8 healthy controls |
9-fold increase expression during migraine attack. | Andersen et al. (2016) [1] |
| Peripheral blood mononuclear cells | 27 patients with EM and 28 with CM–MOH | Higher levels in CM–MOH vs. EM. Positive correlation with CGRP levels. |
Greco et al. (2020) [2] | |
| miR-30a | Serum | Patients with migraine with or without aura (sample size not defined) | Significant lower expression in patients with migraine vs. healthy controls. | Zhai et al. (2018) [3] |
| Other miRNAs | Peripheral blood mononuclear cells | 15 female patients with migraine without aura 13 healthy controls |
Reduced miR-181a, let-7b and miR-22 levels in EM vs. HC. Increased miR-27b levels in EM vs. HC. |
Tafuri et al. (2015) [4] |
| Serum | 8 migraine patients without any medication 12 migraine patients with normal medication habits 8 healthy controls |
Increased miR-29c-5p and miR-26b-3p expression in EM patients. | Andersen et al. (2016) [1] | |
| Serum | 30 patients with EM 30 healthy controls |
Increased expression of miR-155, miR-126, and let-7 in EM vs. HC. | Chen et al. (2018) [5] |
| Treatment | Posology and Duration | Population | miRNAs Modifications and Timing | Reference |
|---|---|---|---|---|
| Detoxification | 7-day standardized detoxification protocol in hospitalized patients: abrupt withdrawal of overused drugs associated to intravenous therapy twice daily with isotonic 0.9% NaCl saline 500 mL + cyanocobalamin 2500 mcg + folic acid 0.70 mg + nicotinamide 12 mg + ascorbic acid 150 mg + sodic glutathione 600 mg + delorazepam 0.5 mg | 28 patients with CM–MOH | Significant reduction of miR-382-5p and miR-34a-5p two months after detoxification in CM–MOH. | Greco et al. (2020) [2] |
| Erenumab 70 mg | One administration s.c. every 28 days for a total of three administrations | 7 patients with CM 33 patients with CM–MOH |
Reduction of miR-382-5p and miR-34a-5p in the overall study population after three months of treatment with erenumab. No differences between 30% Responders and NON-responders after three months of erenumab treatment. |
De Icco et al. (2020) [6] |
| NSAIDs and long-term magnesium | Chronic treatment with magnesium (400 mg/day for three months) + Abortive treatment with acetaminophen (15 mg/kg) or ibuprofen (10 mg/kg) | 24 children and adolescents affected by migraine without aura divided into two groups (treated, and untreated) and 12 healthy controls | Decreased expression of miR-34a-5p in migraine patients treated with NSAIDs and long-term magnesium vs. untreated migraine patients. Decreased expression in healthy controls vs. untreated migraine patients. Comparable expression between migraine patients treated with NSAIDs and long-term magnesium and healthy controls. |
Gallelli et al. (2019) [7] |
| Biphasic ketogenic diet | Phase 1: 3 weeks with <30 g of carbohydrates Phase 2: 3 weeks with <120 g of carbohydrates |
6 female obese patients with migraine | Reduction of has-miR-590-5p and has-miR-660-3p expression after a 6-week ketogenic diet | Cannataro et al. (2020) [8] |
2. miR-382-5p and miR-34a-5p in Migraine
3. miR-382-5p and miR-34a-5p in Pain and Putative Mechanisms
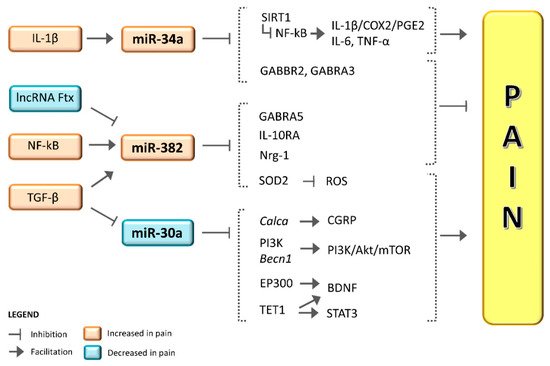
4. Role of miR-30a in Migraine and Pain
Conclusion
References
- Andersen, H.H.; Duroux, M.; Gazerani, P. Serum MicroRNA Signatures in Migraineurs During Attacks and in Pain-Free Periods. Mol. Neurobiol. 2016, 53, 1494–1500.
- Greco, R.; De Icco, R.; Demartini, C.; Zanaboni, A.M.; Tumelero, E.; Sances, G.; Allena, M.; Tassorelli, C. Plasma levels of CGRP and expression of specific microRNAs in blood cells of episodic and chronic migraine subjects: Towards the identification of a panel of peripheral biomarkers of migraine? J. Headache Pain 2020, 21, 122.
- Zhai, Y.; Zhu, Y.Y. MiR-30a relieves migraine by degrading CALCA. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2022–2028.
- Tafuri, E.; Santovito, D.; De Nardis, V.; Marcantonio, P.; Paganelli, C.; Affaitati, G.; Bucci, M.; Mezzetti, A.; Giamberardino, M.A.; Cipollone, F. MicroRNA profiling in migraine without aura: Pilot study. Ann. Med. 2015, 47, 468–473.
- Cheng, C.Y.; Chen, S.P.; Liao, Y.C.; Fuh, J.L.; Wang, Y.F.; Wang, S.J. Elevated circulating endothelial-specific microRNAs in migraine patients: A pilot study. Cephalalgia 2018, 38, 1585–1591.
- De Icco, R.; Fiamingo, G.; Greco, R.; Bottiroli, S.; Demartini, C.; Zanaboni, A.M.; Allena, M.; Guaschino, E.; Martinelli, D.; Putortì, A.; et al. Neurophysiological and biomolecular effects of erenumab in chronic migraine: An open label study. Cephalalgia 2020, 40, 1336–1345.
- Gallelli, L.; Cione, E.; Peltrone, F.; Siviglia, S.; Verano, A.; Chirchiglia, D.; Zampogna, S.; Guidetti, V.; Sammartino, L.; Montana, A.; et al. Hsa-miR-34a-5p and hsa-miR-375 as Biomarkers for Monitoring the Effects of Drug Treatment for Migraine Pain in Children and Adolescents: A Pilot Study. J. Clin. Med. 2019, 8, 928.
- Cannataro, R.; Caroleo, M.C.; Siniscalchi, A.; Gallelli, L.; De Sarro, G.; Cione, E. Ketogenic Diet Modifies the Expression of MicroRNAs Linked to Migraine. Acta Sci. Nutr. Health 2020, 4, 34–41.
- Yamanaka, G.; Suzuki, S.; Morishita, N.; Takeshita, M.; Kanou, K.; Takamatsu, T.; Suzuki, S.; Morichi, S.; Watanabe, Y.; Ishida, Y.; et al. Role of neuroinflammation and blood-brain barrier permutability on migraine. Int. J. Mol. Sci. 2021, 22, 8929.
- Welch, K.M.A.; Chabi, E.V.A.; Bartosh, K.; Achar, V.S.; Meyer, J.S. Cerebrospinal Fluid Y Aminobutyric Acid Levels in Migraine. BMJ 1975, 516–517.
- Marukawa, H.; Shimomura, T.; Takahashi, K. Salivary substance P, 5-hydroxytryptamine, and γ-aminobutyric acid levels in migraine and tension-type headache. Headache 1996, 36, 100–104.
- Abouheif, M.M.; Nakasa, T.; Shibuya, H.; Niimoto, T.; Kongcharoensombat, W.; Ochi, M. Silencing microRNA-34a inhibits chondrocyte apoptosis in a rat osteoarthritis model in vitro. Rheumatology 2010, 49, 2054–2060.
- Endisha, H.; Datta, P.; Sharma, A.; Nakamura, S.; Rossomacha, E.; Younan, C.; Ali, S.A.; Tavallaee, G.; Lively, S.; Potla, P.; et al. MicroRNA-34a-5p Promotes Joint Destruction During Osteoarthritis. Arthritis Rheumatol. 2021, 73, 426–439.
- Cosín-Tomás, M.; Antonell, A.; Lladó, A.; Alcolea, D.; Fortea, J.; Ezquerra, M.; Lleó, A.; Martí, M.J.; Pallàs, M.; Sanchez-Valle, R.; et al. Plasma miR-34a-5p and miR-545-3p as Early Biomarkers of Alzheimer’s Disease: Potential and Limitations. Mol. Neurobiol. 2017, 54, 5550–5562.
- Tazawa, H.; Tsuchiya, N.; Izumiya, M.; Nakagama, H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc. Natl. Acad. Sci. USA 2007, 104, 15472–15477.
- Zarone, M.R.; Misso, G.; Grimaldi, A.; Zappavigna, S.; Russo, M.; Amler, E.; Di Martino, M.T.; Amodio, N.; Tagliaferri, P.; Tassone, P.; et al. Evidence of novel miR-34a-based therapeutic approaches for multiple myeloma treatment. Sci. Rep. 2017, 7, 17949.
- Zanette, D.L.; Rivadavia, F.; Molfetta, G.A.; Barbuzano, F.G.; Proto-Siqueira, R.; Falcão, R.P.; Zago, M.A.; Silva, W.A. miRNA expression profiles in chronic lymphocytic and acute lymphocytic leukemia. Braz. J. Med. Biol. Res. 2007, 40, 1435–1440.
- Raver-Shapira, N.; Marciano, E.; Meiri, E.; Spector, Y.; Rosenfeld, N.; Moskovits, N.; Bentwich, Z.; Oren, M. Transcriptional Activation of miR-34a Contributes to p53-Mediated Apoptosis. Mol. Cell 2007, 26, 731–743.
- Chang, T.C.; Wentzel, E.A.; Kent, O.A.; Ramachandran, K.; Mullendore, M.; Lee, K.H.; Feldmann, G.; Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J.; et al. Transactivation of miR-34a by p53 Broadly Influences Gene Expression and Promotes Apoptosis. Mol. Cell 2007, 26, 745–752.
- Ashina, M.; Terwindt, G.M.; Al-Karagholi, M.A.M.; de Boer, I.; Lee, M.J.; Hay, D.L.; Schulte, L.H.; Hadjikhani, N.; Sinclair, A.J.; Ashina, H.; et al. Migraine: Disease characterisation, biomarkers, and precision medicine. Lancet 2021, 397, 1496–1504.
- Jiang, P.; Liu, R.; Zheng, Y.; Liu, X.; Chang, L.; Xiong, S.; Chu, Y. MiR-34a inhibits lipopolysaccharide-induced inflammatory response through targeting Notch1 in murine macrophages. Exp. Cell Res. 2012, 318, 1175–1184.
- Mathé, E.; Nguyen, G.H.; Funamizu, N.; He, P.; Moake, M.; Croce, C.M.; Hussain, S.P. Inflammation regulates microRNA expression in cooperation with p53 and nitric oxide. Int. J. Cancer 2012, 131, 760–765.
- Tian, F.; Wang, J.; Zhang, Z.; Yang, J. LncRNA SNHG7/miR-34a-5p/SYVN1 axis plays a vital role in proliferation, apoptosis and autophagy in osteoarthritis. Biol. Res. 2020, 53, 9.
- Yan, S.; Wang, M.; Zhao, J.; Zhang, H.; Zhou, C.; Jin, L.; Zhang, Y.; Qiu, X.; Ma, B.; Fan, Q. MicroRNA-34a affects chondrocyte apoptosis and proliferation by targeting the SIRT1/p53 signaling pathway during the pathogenesis of osteoarthritis. Int. J. Mol. Med. 2016, 38, 201–209.
- Brinjikji, W.; Diehn, F.E.; Jarvik, J.G.; Carr, C.M.; Kallmes, D.F.; Murad, M.H.; Luetmer, P.H. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: A systematic review and meta-analysis. Am. J. Neuroradiol. 2015, 36, 2394–2399.
- Zhang, H.; Zhang, X.; Zong, D.; Ji, X.; Jiang, H.; Zhang, F.; He, S. miR-34a-5p up-regulates the IL-1β/COX2/PGE2 inflammation pathway and induces the release of CGRP via inhibition of SIRT1 in rat trigeminal ganglion neurons. FEBS Open Bio 2021, 11, 300–311.
- Zhang, H.; He, S.D.; Zong, D.D.; Zhang, X.M.; Luo, J.; Zheng, J.K. Effects of electroacupuncture on miR-34a-5p/SIRT1 signaling in the trigeminal ganglion of rats with migraine. Zhen Ci Yan Jiu 2020, 45, 868–874.
- Chen, S.; Gu, Y.; Dai, Q.; He, Y.; Wang, J. Spinal miR-34a regulates inflammatory pain by targeting SIRT1 in complete Freund’s adjuvant mice. Biochem. Biophys. Res. Commun. 2019, 516, 1196–1203.
- Yang, H.; Zhang, W.; Pan, H.; Feldser, H.G.; Lainez, E.; Miller, C.; Leung, S.; Zhong, Z.; Zhao, H.; Sweitzer, S.; et al. SIRT1 Activators Suppress Inflammatory Responses through Promotion of p65 Deacetylation and Inhibition of NF-κB Activity. PLoS ONE 2012, 7, e46364.
- Brandenburger, T.; Johannsen, L.; Prassek, V.; Kuebart, A.; Raile, J.; Wohlfromm, S.; Köhrer, K.; Huhn, R.; Hollmann, M.W.; Hermanns, H. MiR-34a is differentially expressed in dorsal root ganglia in a rat model of chronic neuropathic pain. Neurosci. Lett. 2019, 708, 134365.
- Madrigal, M.P.; Portalés, A.; SanJuan, M.P.; Jurado, S. Postsynaptic SNARE Proteins: Role in Synaptic Transmission and Plasticity. Neuroscience 2019, 420, 12–21.
- Quintas, M.; Neto, J.L.; Sequeiros, J.; Sousa, A.; Pereira-Monteiro, J.; Lemos, C.; Alonso, I. Going Deep into Synaptic Vesicle Machinery Genes and Migraine Susceptibility—A Case-Control Association Study. Headache 2020, 60, 2152–2165.
- Hung, K.L.; Wang, S.J.; Wang, Y.C.; Chiang, T.R.; Wang, C.C. Upregulation of presynaptic proteins and protein kinases associated with enhanced glutamate release from axonal terminals (synaptosomes) of the medial prefrontal cortex in rats with neuropathic pain. Pain 2014, 155, 377–387.
- Ishizaki, K.; Takeshima, T.; Fukuhara, Y.; Araki, H.; Nakaso, K.; Kusumi, M.; Nakashima, K. Increased plasma transforming growth factor-β1 in migraine. Headache 2005, 45, 1224–1228.
- Bø, S.H.; Davidsen, E.M.; Gulbrandsen, P.; Dietrichs, E.; Bovim, G.; Stovner, L.J.; White, L.R. Cerebrospinal fluid cytokine levels in migraine, tension-type headache and cervicogenic headache. Cephalalgia 2009, 29, 365–372.
- Lin, C.A.; Duan, K.Y.; Wang, X.W.; Zhang, Z.S. Study on the role of Hsa-miR-382-5p in epidural fibrosis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3663–3668.
- Rossi, C.; Zini, R.; Rontauroli, S.; Ruberti, S.; Prudente, Z.; Barbieri, G.; Bianchi, E.; Salati, S.; Genovese, E.; Bartalucci, N.; et al. Role of TGF-β1/miR-382-5p/SOD2 axis in the induction of oxidative stress in CD34+ cells from primary myelofibrosis. Mol. Oncol. 2018, 12, 2102–2123.
- Kriegel, A.J.; Fang, Y.; Liu, Y.; Tian, Z.; Mladinov, D.; Matus, I.R.; Ding, X.; Greene, A.S.; Liang, M. MicroRNA-target pairs in human renal epithelial cells treated with transforming growth factor β1: A novel role of miR-382. Nucleic Acids Res. 2010, 38, 8338–8347.
- Kato, M.; Zhang, J.; Wang, M.; Lanting, L.; Yuan, H.; Rossi, J.J.; Natarajan, R. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-β-induced collagen expression via inhibition of E-box repressors. Proc. Natl. Acad. Sci. USA 2007, 104, 3432–3437.
- Yorns, W.R.; Hardison, H.H. Mitochondrial dysfunction in migraine. Semin. Pediatr. Neurol. 2013, 20, 188–193.
- Borkum, J.M. Migraine Triggers and Oxidative Stress: A Narrative Review and Synthesis. Headache 2016, 56, 12–35.
- Li, R.; Liu, Y.; Chen, N.; Zhang, Y.; Song, G.; Zhang, Z. Valproate attenuates nitroglycerin-induced trigeminovascular activation by preserving mitochondrial function in a Rat model of migraine. Med. Sci. Monit. 2016, 22, 3229–3237.
- Xiang, W.; Jiang, L.; Zhou, Y.; Li, Z.; Zhao, Q.; Wu, T.; Cao, Y.; Zhou, J. The lncRNA Ftx/miR-382-5p/Nrg1 axis improves the inflammation response of microglia and spinal cord injury repair. Neurochem. Int. 2021, 143, 104929.
- Wan, C.; Xu, Y.; Cen, B.; Xia, Y.; Yao, L.; Zheng, Y.; Zhao, J.; He, S.; Chen, Y. Neuregulin1-ErbB4 Signaling in Spinal Cord Participates in Electroacupuncture Analgesia in Inflammatory Pain. Front. Neurosci. 2021, 15, 636348.
- Kataria, H.; Alizadeh, A.; Karimi-Abdolrezaee, S. Neuregulin-1/ErbB network: An emerging modulator of nervous system injury and repair. Prog. Neurobiol. 2019, 180, 101643.
- Wang, G.; Dai, D.; Chen, X.; Yuan, L.; Zhang, A.; Lu, Y.; Zhang, P. Upregulation of neuregulin-1 reverses signs of neuropathic pain in rats. Int. J. Clin. Exp. Pathol. 2014, 7, 5916–5921.
- Alizadeh, A.; Dyck, S.M.; Kataria, H.; Shahriary, G.M.; Nguyen, D.H.; Santhosh, K.T.; Karimi-Abdolrezaee, S. Neuregulin-1 positively modulates glial response and improves neurological recovery following traumatic spinal cord injury. Glia 2017, 65, 1152–1175.
- Reuter, U.; Chiarugi, A.; Bolay, H.; Moskowitz, M.A. Nuclear factor-κB as a molecular target for migraine therapy. Ann. Neurol. 2002, 51, 507–516.
- Greco, R.; Tassorelli, C.; Cappelletti, D.; Sandrini, G.; Nappi, G. Activation of the Transcription Factor NF-κB in the nucleus trigeminalis caudalis in an animal model of migraine. Neurotoxicology 2005, 26, 795–800.
- Sarchielli, P.; Floridi, A.; Mancini, M.L.; Rossi, C.; Coppola, F.; Baldi, A.; Pini, L.A.; Calabresi, P. NF-κB activity and iNOS expression in monocytes from internal jugular blood of migraine without aura patients during attacks. Cephalalgia 2006, 26, 1071–1079.
- Novák, J.; Olejníčková, V. microRNA: Basic Science; Springer International Publishing: Cham, Switzerland, 2015; Volume 887, pp. 79–100.
- Wang, X.; Xue, N.; Zhao, S.; Shi, Y.; Ding, X.; Fang, Y. Upregulation of miR-382 contributes to renal fibrosis secondary to aristolochic acid-induced kidney injury via PTEN signaling pathway. Cell Death Dis. 2020, 11, 620.
- Zhu, Q.; Li, H.; Li, Y.; Jiang, L. MicroRNA-30a functions as tumor suppressor and inhibits the proliferation and invasion of prostate cancer cells by down-regulation of SIX1. Hum. Cell 2017, 30, 290–299.
- Shepard, A.; Hoxha, S.; Troutman, S.; Harbaugh, D.; Kareta, M.S.; Kissil, J.L. Transcriptional regulation of miR-30a by YAP impacts PTPN13 and KLF9 levels and Schwann cell proliferation. J. Biol. Chem. 2021, 297, 100962.
- Li, J.; Xie, Y.; Li, L.; Li, X.; Shen, L.; Gong, J.; Zhang, R. MicroRNA-30a Modulates Type I Interferon Responses to Facilitate Coxsackievirus B3 Replication Via Targeting Tripartite Motif Protein 25. Front. Immunol. 2021, 11, 603437.
- Li, Y.; Zhang, J.; Liu, Y.; Zhang, B.; Zhong, F.; Wang, S.; Fang, Z. MiR-30a-5p confers cisplatin resistance by regulating IGF1R expression in melanoma cells. BMC Cancer 2018, 18, 404.
- Shi, S.; Yu, L.; Zhang, T.; Qi, H.; Xavier, S.; Ju, W.; Bottinger, E. Smad2-Dependent Downregulation of miR-30 Is Required for TGF-β-Induced Apoptosis in Podocytes. PLoS ONE 2013, 8, e75572.
- Volkmann, I.; Kumarswamy, R.; Pfaff, N.; Fiedler, J.; Dangwal, S.; Holzmann, A.; Batkai, S.; Geffers, R.; Lother, A.; Hein, L.; et al. MicroRNA-mediated epigenetic silencing of sirtuin1 contributes to impaired angiogenic responses. Circ. Res. 2013, 113, 997–1003.
- Chen, H.; Wang, Y.; Xu, Y.; Wang, G.N. Overexpression of miR-30a attenuates neuropathic pain by targeting SOCS1 in rats with chronic constriction injury. Int. J. Clin. Exp. Pathol. 2016, 9, 1258–1266.
- Tan, M.; Shen, L.; Hou, Y. Epigenetic modification of BDNF mediates neuropathic pain via miR-30a-3p/EP300 axis in CCI rats. Biosci. Rep. 2020, 40, BSR20194442.
- Zhong, M.; Bian, Z.; Wu, Z. MiR-30a suppresses cell migration and invasion through downregulation of PIK3CD in colorectal carcinoma. Cell. Physiol. Biochem. 2013, 31, 209–218.
- Zhang, L.; Cheng, R.; Huang, Y. MiR-30a inhibits BECN1-mediated autophagy in diabetic cataract. Oncotarget 2017, 8, 77360–77368.
- Chen, S.-P.; Zhou, Y.-Q.; Liu, D.-Q.; Zhang, W.; Manyande, A.; Guan, X.-H.; Tian, Y.; Ye, D.-W.; Omar, D.M. PI3K/Akt Pathway: A Potential Therapeutic Target for Chronic Pain. Curr. Pharm. Des. 2017, 23, 1860–1868.
- Liu, W.; Lv, Y.; Ren, F. PI3K/Akt Pathway is Required for Spinal Central Sensitization in Neuropathic Pain. Cell. Mol. Neurobiol. 2018, 38, 747–755.
- Guo, J.-R.; Wang, H.; Jin, X.-J.; Jia, D.-L.; Zhou, X.; Tao, Q. Effect and mechanism of inhibition of PI3K/Akt/mTOR signal pathway on chronic neuropathic pain and spinal microglia in a rat model of chronic constriction injury. Oncotarget 2017, 8, 52923–52934.
- Zhang, S.; Liu, H.; Liu, Y.; Zhang, J.; Li, H.; Liu, W.; Cao, G.; Xv, P.; Zhang, J.; Lv, C.; et al. miR-30a as potential therapeutics by targeting tet1 through regulation of Drp-1 promoter hydroxymethylation in idiopathic pulmonary fibrosis. Int. J. Mol. Sci. 2017, 18, 633.
- Hsieh, M.C.; Lai, C.Y.; Ho, Y.C.; Wang, H.H.; Cheng, J.K.; Chau, Y.P.; Peng, H.Y. Tet1-dependent epigenetic modification of BDNF expression in dorsal horn neurons mediates neuropathic pain in rats. Sci. Rep. 2016, 6, 37411.
- Pan, Z.; Xue, Z.Y.; Li, G.F.; Sun, M.L.; Zhang, M.; Hao, L.Y.; Tang, Q.Q.; Zhu, L.J.; Cao, J.L. DNA Hydroxymethylation by Ten-eleven Translocation Methylcytosine Dioxygenase 1 and 3 Regulates Nociceptive Sensitization in a Chronic Inflammatory Pain Model. Anesthesiology 2017, 127, 147–163.





