Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maxence Lejars | + 3157 word(s) | 3157 | 2021-12-23 08:55:59 | | | |
| 2 | Camila Xu | Meta information modification | 3157 | 2021-12-27 06:33:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lejars, M. RNase III. Encyclopedia. Available online: https://encyclopedia.pub/entry/17549 (accessed on 13 January 2026).
Lejars M. RNase III. Encyclopedia. Available at: https://encyclopedia.pub/entry/17549. Accessed January 13, 2026.
Lejars, Maxence. "RNase III" Encyclopedia, https://encyclopedia.pub/entry/17549 (accessed January 13, 2026).
Lejars, M. (2021, December 24). RNase III. In Encyclopedia. https://encyclopedia.pub/entry/17549
Lejars, Maxence. "RNase III." Encyclopedia. Web. 24 December, 2021.
Copy Citation
RNase III endoribonucleases cleave dsRNA and are conserved from bacteria (e.g., RNase III) to eukaryotes (e.g., Rnt1, Drosha and Dicer) both in terms of structure and function.
RNase III
ribosome biogenesis
bacteria
eukaryotes
1. Introduction
Universally conserved, the ribosome is a complex ribonucleoparticle that acts as the catalyst for protein synthesis. Ribosomal RNAs (rRNAs) and ribosomal proteins are assembled in a stepwise manner involving a plethora of protein and RNA effectors. As one of the most elaborate biological machines, the ribosome remains a major object of study in regard to its complex nature and its heterogeneity in cells [1]. In prokaryotes and eukaryotes, ribosomal biogenesis relies on the transcription of precursors rRNAs, which are processed into mature rRNAs [2][3]. The complexity of the step-by-step process of maturation reveals a need for precision in the timing as well as robustness in the synthesis of rRNAs. In particular, rRNAs adopt complex structures, which are achieved through double-stranded RNA (dsRNA) motifs [4] and which are essential for both catalytic and structural integrity of the mature ribosome [5].
In cells, RNAs are protected by RNA-binding proteins (RBP) and RNA chaperones and are processed by ribonucleases (RNases). Although naked single-stranded RNAs can be cleaved by various RNases, the processing of stable dsRNA structures requires specialized RNase III domain (RIIID)-containing enzymes. RNase IIIs are endoribonucleases cleaving dsRNA conserved from bacteria (e.g., RNase III) to eukaryotes (e.g., Rnt1, Drosha and Dicer) both in terms of structure and function [6][7][8][9][10][11]. RNase III was first identified for its role in the initial step of rRNA maturation in the model organism Escherichia coli [12]. Subsequent studies demonstrated that RNase III is also involved in the regulation of gene expression in bacteria and the maturation of non-coding RNAs in eukaryotes.
2. The Founding Member
2.1. The rnc Gene
RNase III was discovered in E. coli (referred hereafter as Ec-RNase III) in 1967 [13] and shown to cleave natural and synthetic dsRNAs in vitro in 1968 [14]. Isolation of an RNase III-deficient derivative of E. coli in 1973 [15] revealed its importance in the initial steps of the maturation of rRNA precursors [12]. Assuming that Ec-RNase III could have an important role in cell physiology, Kindler et al. isolated thermosensitive mutants from an E. coli strain inactivated for RNase I [16]. The clone AB301-105 carrying the rnc105 mutation (Table 1) demonstrated a strongly reduced ability to cleave dsRNA substrates in vitro [15]. The rnc105 mutation was then mapped around 49 min on the chromosome of E. coli, identifying the rnc gene, which was subsequently confirmed to encode Ec-RNase III [17]. Although Ec-RNase III is not essential for growth [18], its inactivation was found to provoke slow growth and sensitivity to heat [19] and osmotic shock [20].
Table 1. Functionally characterized RNase III mutants in E. coli.
| Allele | Mutation | Catalytic Activity | In Vitro dsRNA Binding | References | |
|---|---|---|---|---|---|
| In Vivo | In Vitro | ||||
| E38A | n.d. | weak 1 | high | [21][22] | |
| E38V | no | n.d. | n.d. | [23] | |
| E41A | n.d. | weak 1 | low | [21] | |
| F42G, D or R | no | n.d. | n.d. | [23] | |
| F42M, W | yes | n.d. | yes | [23] | |
| rnc105 | G44D | no | no | no | [24][25] |
| D45A | no | low | yes | [21][23] | |
| D45E or N | n.d. | weak 1 | low | [21] | |
| E65A | no | weak 1 | low | [21][23] | |
| rnc97 | G97E | low | weak 1 | n.d. | [26] |
| E100A | n.d. | weak 1 | low | [21] | |
| D114A | n.d. | weak 1 | low | [21] | |
| E117D | n.d. | weak 1 | yes | [23][27] | |
| rnc70 | E117K, A or Q | no | no | yes | [28][29][30] |
| rnc10 | Q153P | n.d. 2 | weak | n.d. | [31] |
| rnc7 | D155E | n.d. 2 | no | n.d. | [31] |
| rev3 | A211V | n.d. 2 | n.d. | n.d. | [25][32] |
| rnc38 | insertion | no | no | no | [33] |
| rnc40 | insertion | no | no | no | [18] |
| rnc14 | insertion | no | no | no | [18] |
Mutations are either missense, indicated by the amino acid, its number relative to the sequence of Ec-RNase III followed by the replacing amino acid(s) or insertions of fragments derived from transposons and plasmids. 1 In excess of magnesium. 2 Suppressors of cold-sensitive mutant alleles. n.d. not determined.
Ec-RNase III is co-expressed with the essential GTPase Era within the rnc-era-recO-pdxJ-acpS operon. The recO gene encodes a DNA repair protein while the pdxJ and acpS genes, also transcribed from their own promoters, encode the pyridoxine synthase PdxJ, essential for growth in the absence of pyridoxine and the essential holo-acyl-carrier-protein synthase AcpS, respectively. Hence, as demonstrated by the absence of an rnc deletion mutant in the Keio collection [34], inactivating Ec-RNase III likely lead to polar effects on the expression of the gene era, which was shown to be essential but is also toxic when overexpressed [35]. Interestingly, insertion mutants of RNase III (rnc14 and rnc38, carrying large inserts containing transposons and fragments of plasmid) seem to provide the correct compensation of era expression. In addition, RNase III cleaves its own mRNA in its 5′ untranslated region (UTR), leading to the destabilization of both rnc and era mRNAs [36][37].
Bacterial RNase III carry two distinct domains: an N-terminal region containing the characteristic catalytic core of RNase III (RIIID) (amino acids 6–128) and the double-stranded RNA (dsRNA)-binding domain (dsRBD) in the C-terminal region (amino acids 155–225) (Figure 1). Various mutations were characterized, which disrupt the catalytic activity of Ec-RNase III (with or without affecting its binding affinity) or abolish its expression (Table 1). The mutations which affect catalytic ability are all located in the RIIID with the exception of rnc7 (D155E) and Q153 located in the dsRBD and the linker, respectively [31]. Importantly, as shown for a few mutants (e.g., rnc70), the catalytic activity of RNase III can be lost without affecting its binding to dsRNA. Moreover, the rnc70 mutant was shown to be dominant over the wt allele in the regulation of the N mRNA from the lambda phage, suggesting that dsRNA binding and cleavage are independent functions of Ec-RNase III [30].
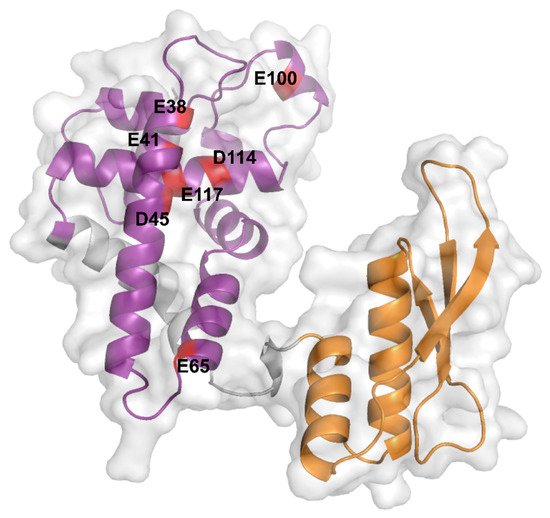
Figure 1. AlphaFold prediction of the Ec-RNase III structure. Ec-RNase III structure predicted by the AlphaFold program (AF-P0A7Y0-F1, https://alphafold.ebi.ac.uk, accessed on the 11 October 2021). The N-terminal RIIID (6-128) and C-terminal dsRBD (155-225) are depicted in purple and orange, respectively. Critical negatively charged residues E38, E41, D45, E65, E100, D114 and E117, which are highly conserved among bacterial RNase III enzymes, are highlighted in red on the structure of the Ec-RNase III. The representation was generated using the PyMol software version 2.5.1.
2.2. How Does RNase III Work?
2.2.1. Structure
The dimerization of the active Ec-RNase III complex relies on the interaction of two RIIIDs to form a single processing center accommodating two divalent metallic ions, with a preference for magnesium [29]. The interaction of the two RIIIDs, achieved through a ball and socket junction, is essential for the correct positioning of the cleavage sites [23]. In the UniProt database (https://www.uniprot.org [38], accessed on the 11 October 2021), RNase III structures from various bacterial species (i.e., Aquifex aeolicus O67082, Campylobacter jejuni Q9PM40, Thermotoga maritima Q9X0I6 and Mycobacterium tuberculosis P9WH03) are available but not from E. coli. These structures revealed that the RIIID is composed of seven α-helices and adopts a unique fold while the dsRBD adopts an α-β-β-β-α topology, as found in other dsRBD containing enzymes [39]. While no complete structure of Ec-RNase III is currently available, Ec-RNase III dsRBD was solved by NMR spectroscopy showing a typical α-β-β-β-α topology [40]. Furthermore, structure prediction performed recently through the AlphaFold program (https://alphafold.ebi.ac.uk, accessed on the 11 October 2021) [41][42] supports a common structure between Ec-RNase III (P0A7Y0) and previously obtained RNase III structures in bacteria. In addition, important amino acid residues have been identified from a wide range of Ec-RNase III mutants (Table 1) and include residues E38, E41, F42, G44, D45, E65, G97, E100, D114, E117 in the RIIID, Q153 in the linker and D155 and A211 in the dsRBD. In particular, negatively charged residues E38, E41, D45, E65, E100, D114 and E117 are highly conserved among bacterial RNase III enzymes [21] (Figure 1).
2.2.2. Mechanism
Recruitment of an RNase III dimer is initiated by the interaction of a single dsRBD with a target dsRNA. The binding induces a conformational change of the dsRBD, bringing it closer to the dsRNA which facilitates the symmetric recruitment of the second dsRBD [43]. Upon binding, the second dsRBD is reoriented which renders the catalytic core functional. Of note, if the dsRNA structure does not correctly accommodate the dimer, a non-catalytic RNase III complex can form and the regulatory outcome of this interaction remains unclear. At the molecular level, RNA cleavage relies on a nucleophilic attack, allowing the hydrolysis (supposedly simultaneous) of the phosphodiester bond generating 3′-hydroxyl and 5′-phosphate ends on both strands but which are staggered by two bases on one strand of the dsRNA compared to the other (Figure 2). The release of the generated cleavage products remains poorly characterized but was shown to be the limiting step of the reaction likely due to the requirement for a second change in the conformation of the dimer to release the processed RNA fragments [44].
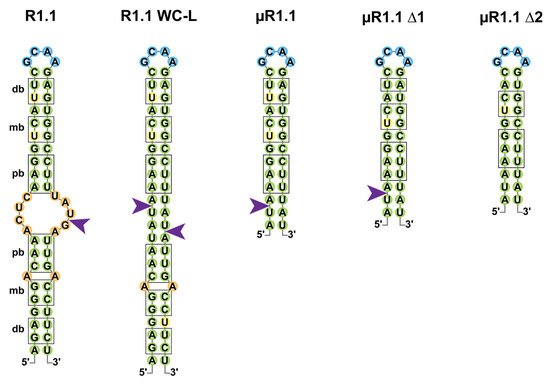
Figure 2. Cleavage of the T7 R1.1 RNA and its derivatives by Ec-RNase III. Secondary structures of RNase III target R1.1 RNA from the T7 phage and its artificial derivatives (R1.1 WC-L, µR1.1, µR1.1 Δ1 and µR1.1 Δ2) are represented in two dimensions as in [29][45]. Base pairs are represented in green; bulges and mismatches are represented in orange, loops in blue and uridine residues involved in wobble G-U base pairing in yellow. Proximal (pb), middle (mb) and distal (db) boxes are shown outlined in black and RNase III single-strand cleavage sites are represented by a purple arrow.
If the early studies of Ec-RNase III suggested that binding and (single or double-stranded) cleavage was not random, criteria for RNase III target selection remain poorly understood. In the pre-mRNA of the T7 phage, multiple single-stranded cleavages sites were identified, of which the “R1.1” cleavage, located at the 5887th nucleotide (nt) of the T7 phage pre-mRNA, drives the maturation and protection of T7 mRNAs (Figure 2) [46][47]. R1.1 is formed by a 49 nts long stem carrying symmetrical sets of proximal (relative to the cleavage site) boxes (pb, 4 nts), middle boxes (mb, 2 nts) and distal boxes (db, 2 nts) for each of the monomers to bind the dsRNA [11][48]. R1.1 became a model to understand the properties of RNase III binding and cleavage and a mutant named R1.1 WC-L, in which full complementarity is forced in the stem enabled the double-stranded cleavage of the RNA [29]. Shortening of the R1.1 stem revealed that a single set of boxes in a 12 base pair (bp) stem with a tetraloop is sufficient for single-strand cleavage by Ec-RNase III (µR1.1 Δ1) while the cleavage is lost upon removal of the last bp of the distal box (µR1.1 Δ2) [45]. It is noteworthy that the recent identification of thousands of staggered double-stranded Ec-RNase III cleavage sites in E. coli suggests that RNase III binding sites do not share a strong consensus sequence but likely depend on the structure of the targeted stem loop [49].
2.2.3. Physiological Roles of RNase III
In addition to its role in rRNA maturation, the importance of bacterial RNase III was highlighted through transcriptomic studies performed in diverse organisms, including but not limited to E. coli [49][50][51][52], Streptomyces coelicolor [53], Staphylococcus aureus [54], Synechococcus sp. PCC7002 [55] or Rhodobacter sphaeroides [56]. These studies demonstrate the pleiotropic role of RNase III in the control of gene expression and a comparison of the genes affected by RNase III inactivation in these organisms would be informative about the distribution and conservation (or not) of targets. In E. coli, RNase III is involved in the destabilization of numerous RNAs. For example, Ec-RNase III cleaves its own mRNA in the 5′UTR of the rnc-era mRNA, which destabilizes the whole transcript (Figure 3) [37]. Ec-RNase III can cleave in between coding sequences within polycistronic mRNAs such as rpsO-pnp, encoding the ribosomal protein S15 and the exoribonuclease PNPase. This cleavage leads to the destabilization of pnp mRNA without affecting the expression of rpsO (Figure 3). Cleavages within coding sequences were also found as in the arfA mRNA (Figure 3), encoding the alternative ribosome rescue factor ArfA, thus revealing a positive role of Ec-RNase III in an alternative pathway to rescue stalled ribosomes upon mRNA truncation [57]. While targets of RNase III in bacteria are usually expected to be negatively regulated, maturation can also lead to positive regulation as in the case of the pre-rRNA (see Section 4.1). RNase III is also involved in intermolecular dsRNA cleavages (i.e., where the dsRNA is composed of two distinct molecules) as in the case of regulatory RNAs bound to their targets. For the small RNA RhyB, binding to the sodB mRNA, encoding the superoxide dismutase FeSOD, RNase III cleavage leads to the degradation of both RNAs [58] while, on the other hand, the cleavage of the antisense RNA (asRNA) ArrS bound to the gadE mRNA leads to increased translation of GadE, an acid response transcriptional factor [59]. These and other examples demonstrate the pleiotropic functions of RNase III in bacterial physiology. For example, in the adaptation phase following an osmotic shock RNase III activity is repressed, which allows stabilization of proP (Figure 3), proU and betT mRNAs encoding proteins involved in the import of osmoprotectants [60][61][62]. Furthermore, RNase III was shown to be involved in thermotolerance [19], motility [63] and aminoglycoside resistance [64]. In other bacteria, RNase III was shown to be important for a whole range of functions, including but not limited to methionine biosynthesis in S. aureus [65] and cell wall homeostasis in Pseudomonas putida [66] and to be involved in virulence in Enterococcus faecalis [67], Listeria monocytogenes [68], S. aureus [54] or Campylobacter jejuni [69].
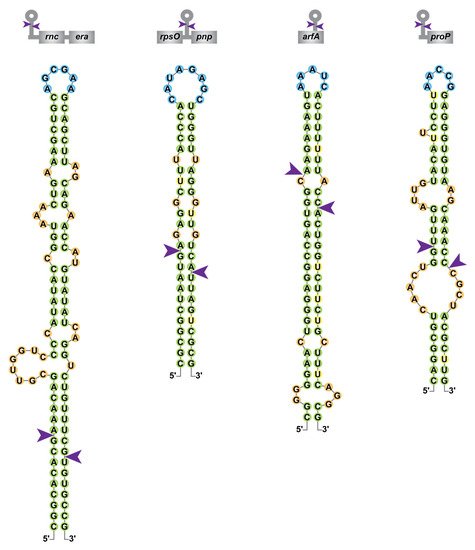
Figure 3. Diversity of RNase III cleavage sites within E. coli mRNAs. Secondary structure predictions of Ec-RNase III targets within the rnc-era, rpsO-pnp, arfA and proP mRNAs were obtained from [49][55][62][70] and color-coded as in Figure 2. A schematic representation of the targeted mRNAs is presented on top of each RNA structure with coding sequences in grey boxes.
Although the majority of characterized RNase III target sites in E. coli likely result from intramolecular dsRNA, it was recently shown that among the thousands of in vivo Ec-RNase III cleavage sites identified, around 40% are singletons, in the sense that there is no obvious staggered second cleavage site. Hence, this suggests that they either represent single-stranded cleavages or arise from intermolecular interactions (i.e., the second single-strand cleavage is located in a second molecule thus a complex analysis is required to predict candidate dsRNA partners) [49]. The plasticity of RNase III binding and cleavage sites in E. coli, as illustrated in Figure 3 for the rnc-era, rpsO-pnp, arfA and proP mRNAs, may provide an explanation for the abundance of putative RNase III cleavage sites (identified by transcriptomic approaches but not yet validated as direct targets) and is consistent with a larger role of RNase III in the regulation of gene expression.
3. RNase III Are Everywhere
RNase III enzymes are widely conserved and have been categorized into four classes according to their domain composition (Figure 4). The first one includes all bacterial RNase III (e.g., RNase III and Mini-III) and the yeast RNase III (e.g., Rnt1p and Pac1p) carrying an additional N-terminal domain. The second class includes eukaryotic RNase III carrying additional domains (see Section 3.2). The sole members of class III and IV are the eukaryotic Drosha and Dicer, respectively, where the RIIID is part of complex multidomain proteins. Class I and II RNase III enzymes are directly involved in ribosomal biogenesis and carry a single RIIID per monomer. On the contrary, classes III and IV enzymes carry two RIIIDs and their direct involvement in rRNA maturation has yet to be elucidated. In addition, RNase III enzymes were also found in viruses such as the essential class I RNase III in Ambystoma tigrinum virus [70]. Remarkably, RNase III has not been found in archaea where dsRNA cleavage is assured by enzymes belonging to the family of splicing endonucleases [71] which recognize bulge–helix–bulge secondary structure motifs and cut within single-stranded bulges [72].
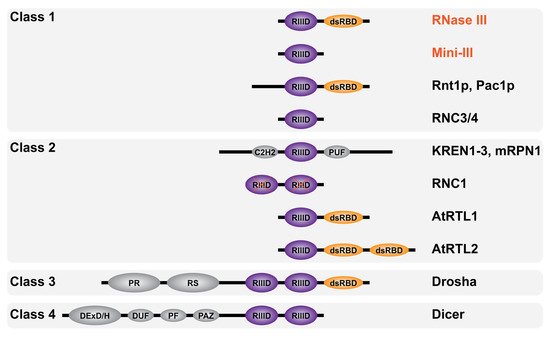
Figure 4. Domain diversity of RNase III enzymes. Schematic representation of RNase III enzymes domain composition categorized by classes as described in the text (not to scale). Bacterial RNase III are in red font and eukaryotic RNase III in black font. The RNase III catalytic domain (RIIID) is in purple and marked with a red X when inactive (in RNC1), the double-stranded RNA binding domain (dsRBD) is in orange while the Zinc finger domain C2H2, RNA-binding domain PUF, proline-rich domain PR, arginine/serine (RS)-rich domain, helicase DExD/H domain, RNA annealing domain DUF, structural domain PF and the anchoring domain PAZ are in gray.
3.1. Bacterial RNase III
The conservation of the RIIID within bacterial genomes allowed the identification of RNase III enzymes in the majority of bacterial species with, so far, the exception of Deinococcus radiodurans [6]. Similar to Ec-RNase III [18], RNase III is not essential in most bacteria (e.g., S. aureus [73], C. jejuni [69], Borrelia burgdorferi [74] or Synechococcus sp. strain PCC 7002 [55]). However, RNase III was shown to be essential in B. subtilis due to its requirement for toxin silencing [75].
To obtain a better understanding of RNase III binding sites and cleavage determinants among species, complementation and substrate specificity assays have often been used. B. subtilis RNase III (referred hereafter as Bs-RNase III) exhibits 36% sequence identity with Ec-RNase III and is able to complement the maturation of rRNAs when expressed in an E. coli rnc mutant [76]. However, although Bs-RNase III can cleave at the same location of some Ec-RNase III substrates in vitro, the contrary is not valid for the Bs-RNase III targets tested [77]. Of note, B. subtilis also contains a shorter form of RNase III that lacks the dsRBD, named Mini-III (hereafter referred to as Bs-Mini-III) which catalyzes the 23S rRNA maturation [78]. Furthermore, while RNase III from Rhodobacter capsulatus can cleave some of Ec-RNase III substrates at the exact position in vitro, the contrary is not true, as Ec-RNase III is unable to process the R. capsulatus pre-23S rRNA [79][80]. In the cyanobacteria Synechococcus sp. strain PCC 7002, three RNase III enzymes were identified, of which one is a homologue to the Bs-Mini-III [55]. Two of them are involved in independent maturation events of the pre-23S rRNA while another participates in plasmid copy number regulation.
3.2. Eukaryotic RNase III
The first eukaryotic RNase III enzymes were identified by sequence comparison with Ec-RNase III. Pairwise comparison of the entire Ec-RNase III revealed 24% identity with Pac1p from the yeast Schizosaccharomyces pombe [81][82] and 25% identity with Rnt1p from the yeast Saccharomyces cerevisiae [83] (as compared to 36% identity between Ec and Bs-RNase III). Analogous to the known function of bacterial RNase III, Pac1p and Rnt1p were also shown to be involved in rRNA maturation [84][85]. Other RNase III members were identified, thanks to their RIIID signature domain, in higher eukaryotes. Drosha [86] and Dicer [87] are involved in different steps of the maturation of micro RNAs (miRNAs) and silencing RNAs (siRNAs) within the RNA interference pathway [88]. Additional eukaryotic RNase III enzymes demonstrating different domain compositions are represented in Figure 4. They include KREN1 to 3 and mRPN1 from Trypanosoma brucei mitochondrial kinetoplast, which contain a C2H2 Zinc finger and whose precise roles remain unclear [89][90], RNC1 in Zea mays chloroplasts, whose two RIIIDs are catalytically inactive [91], AtRTL1/2 class II RNase III-like enzymes in Arabidopsis thaliana nucleus [92][93] and RNC3/4 mini-RNase III-like enzymes in Arabidopsis thaliana chloroplast [94].
References
- Genuth, N.R.; Barna, M. The Discovery of Ribosome Heterogeneity and Its Implications for Gene Regulation and Organismal Life. Mol. Cell 2018, 71, 364–374.
- Braun, F.; Condon, C. RNA Processing. In Encyclopedia of Microbiology; Academic Press: Cambridge, MA, USA, 2019.
- Henras, A.K.; Plisson-Chastang, C.; O’Donohue, M.-F.; Chakraborty, A.; Gleizes, P.-E. An overview of pre-ribosomal RNA processing in eukaryotes. Wiley Interdiscip. Rev. RNA 2015, 6, 225–242.
- Ganser, L.R.; Kelly, M.L.; Herschlag, D.; Al-Hashimi, H.M. The roles of structural dynamics in the cellular functions of RNAs. Nat. Rev. Mol. Cell Biol. 2019, 20, 474–489.
- Steitz, T.A.; Moore, P.B. RNA, the first macromolecular catalyst: The ribosome is a ribozyme. Trends Biochem. Sci. 2003, 28, 411–418.
- Condon, C.; Putzer, H. The phylogenetic distribution of bacterial ribonucleases. Nucleic Acids Res. 2002, 30, 5339–5346.
- Chanfreau, G. Conservation of RNase III processing pathways and specificity in hemiascomycetes. Eukaryot. Cell 2003, 2, 901–909.
- Lamontagne, B.; Larose, S.; Boulanger, J.; Elela, S.A. The RNase III family: A conserved structure and expanding functions in eukaryotic dsRNA metabolism. Curr. Issues Mol. Biol. 2001, 3, 71–78.
- Wu, H.; Xu, H.; Miraglia, L.J.; Crooke, S.T. Human RNase III is a 160-kDa protein involved in preribosomal RNA processing. J. Biol. Chem. 2000, 275, 36957–36965.
- Rotondo, G.; Huang, J.Y.; Frendewey, D. Substrate structure requirements of the Pac1 ribonuclease from Schizosaccharmyces pombe. RNA 1997, 3, 1182–1193.
- Court, D.L.; Gan, J.; Liang, Y.H.; Shaw, G.X.; Tropea, J.E.; Costantino, N.; Waugh, D.S.; Ji, X. RNase III: Genetics and function; structure and mechanism. Annu. Rev. Genet. 2013, 47, 405–431.
- Nikolaev, N.; Schlessinger, D.; Wellauer, P.K. 30 S pre-ribosomal RNA of Escherichia coli and products of cleavage by ribonuclease III: Length and molecular weight. J. Mol. Biol. 1974, 86, 741–748.
- Robertson, H.D.; Webster, R.E.; Zinder, N.D. A nuclease specific for double-stranded RNA. Virology 1967, 32, 718–719.
- Robertson, H.D.; Webster, R.E.; Zinder, N.D. Purification and properties of ribonuclease III from Escherichia coli. J. Biol. Chem. 1968, 243, 82–91.
- Kindler, P.; Keil, T.U.; Hofschneider, P.H. Isolation and characterization of a ribonuclease III deficient mutant of Escherichia coli. Mol. Gen. Genet. 1973, 126, 53–69.
- Gesteland, R.F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J. Mol. Biol. 1966, 16, 67–84.
- Studier, F.W. Genetic mapping of a mutation that causes ribonucleases III deficiency in Escherichia coli. J. Bacteriol. 1975, 124, 307–316.
- Takiff, H.E.; Chen, S.M.; Court, D.L. Genetic analysis of the rnc operon of Escherichia coli. J. Bacteriol. 1989, 171, 2581–2590.
- Apirion, D.; Neil, J.; Watson, N. Consequences of losing ribonuclease III on the Escherichia coli cell. Mol. Gen. Genet. 1976, 144, 185–190.
- Sim, S.-H.; Yeom, J.-H.; Shin, C.; Song, W.-S.; Shin, E.; Kim, H.-M.; Cha, C.-J.; Han, S.H.; Ha, N.-C.; Kim, S.W.; et al. Escherichia coli ribonuclease III activity is downregulated by osmotic stress: Consequences for the degradation of bdm mRNA in biofilm formation. Mol. Microbiol. 2010, 75, 413–425.
- Sun, W.; Li, G.; Nicholson, A.W. Mutational Analysis of the Nuclease Domain of Escherichia coli Ribonuclease III. Identification of Conserved Acidic Residues that Are Important for Catalytic Function in Vitro. Biochemistry 2004, 43, 13054–13062.
- Xiao, J.; Feehery, C.E.; Tzertzinis, G.; Maina, C.V. E. coli RNase III(E38A) generates discrete-sized products from long dsRNA. RNA 2009, 15, 984–991.
- Blaszczyk, J.; Tropea, J.E.; Bubunenko, M.; Routzahn, K.M.; Waugh, D.S.; Court, D.L.; Ji, X. Crystallographic and Modeling Studies of RNase III Suggest a Mechanism for Double-Stranded RNA Cleavage. Structure 2001, 9, 1225–1236.
- Apirion, D.; Watson, N. Mapping and characterization of a mutation in Escherichia coli that reduces the level of ribonuclease III specific for double-stranded ribonucleic acid. J. Bacteriol. 1975, 124, 317–324.
- Nashimoto, H.; Uchida, H. DNA sequencing of the Escherichia coli ribonuclease III gene and its mutations. Mol. Gen. Genet. 1985, 201, 25–29.
- Davidov, Y.; Rahat, A.; Flechner, I.; Pines, O. Characterization of the rnc-97 mutation of RNase III: A glycine to glutamate substitution increases the requirement for magnesium ions. Microbiology 1993, 139, 717–724.
- Sun, W.; Nicholson, A.W. Mechanism of Action of Escherichia coli Ribonuclease III. Stringent Chemical Requirement for the Glutamic Acid 117 Side Chain and Mn2+ Rescue of the Glu117Asp Mutant. Biochemistry 2001, 40, 5102–5110.
- Inada, T.; Kawakami, K.; Chen, S.M.; Takiff, H.E.; Court, D.L.; Nakamura, Y. Temperature-sensitive lethal mutant of era, a G protein in Escherichia coli. J. Bacteriol. 1989, 171, 5017–5024.
- Li, H.; Nicholson, A.W. Defining the enzyme binding domain of a ribonuclease III processing signal. Ethylation interference and hydroxyl radical footprinting using catalytically inactive RNase III mutants. EMBO J. 1996, 15, 1421–1433.
- Dasgupta, S.; Fernandez, L.; Kameyama, L.; Inada, T.; Nakamura, Y.; Pappas, A.; Court, D.L. Genetic uncoupling of the dsRNA-binding and RNA cleavage activities of the Escherichia coli endoribonuclease RNase III--the effect of dsRNA binding on gene expression. Mol. Microbiol. 1998, 28, 629–640.
- Inada, T.; Nakamura, Y. Lethal double-stranded RNA processing activity of ribonuclease III in the absence of SuhB protein of Escherichia coli. Biochimie 1995, 77, 294–302.
- Nashimoto, H.; Miura, A.; Saito, H.; Uchida, H. Suppressors of temperature-sensitive mutations in a ribosomal protein gene, rpsL (S12), of Escherichia coli K12. Mol. Gen. Genet. 1985, 199, 381–387.
- Babitzke, P.; Granger, L.; Olszewski, J.; Kushner, S.R. Analysis of mRNA decay and rRNA processing in Escherichia coli multiple mutants carrying a deletion in RNase III. J. Bacteriol. 1993, 175, 229–239.
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Systems Biol. 2006, 2.
- Zhou, X.; Peters, H.K., 3rd; Li, X.; Costantino, N.; Kumari, V.; Shi, G.; Tu, C.; Cameron, T.A.; Haeusser, D.P.; Vega, D.E.; et al. Overproduction of a Dominant Mutant of the Conserved Era GTPase Inhibits Cell Division in Escherichia coli. J. Bacteriol. 2020, 202.
- Keseler, I.M.; Mackie, A.; Santos-Zavaleta, A.; Billington, R.; Bonavides-Martínez, C.; Caspi, R.; Fulcher, C.; Gama-Castro, S.; Kothari, A.; Krummenacker, M.; et al. The EcoCyc database: Reflecting new knowledge about Escherichia coli K-12. Nucleic Acids Res. 2016, 45, 543–550.
- Bardwell, J.C.; Régnier, P.; Chen, S.M.; Nakamura, Y.; Grunberg-Manago, M.; Court, D.L. Autoregulation of RNase III operon by mRNA processing. EMBO J. 1989, 8, 3401–3407.
- Consortium, T.U. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2020, 49, D480–D489.
- Masliah, G.; Barraud, P.; Allain, F.H.T. RNA recognition by double-stranded RNA binding domains: A matter of shape and sequence. Cell. Mol. Life Sci. 2013, 70, 1875–1895.
- Kharrat, A.; Macias, M.J.; Gibson, T.J.; Nilges, M.; Pastore, A. Structure of the dsRNA binding domain of E. coli RNase III. EMBO J. 1995, 14, 3572–3584.
- Tunyasuvunakool, K.; Adler, J.; Wu, Z.; Green, T.; Zielinski, M.; Žídek, A.; Bridgland, A.; Cowie, A.; Meyer, C.; Laydon, A.; et al. Highly accurate protein structure prediction for the human proteome. Nature 2021.
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589.
- Gan, J.; Tropea, J.E.; Austin, B.P.; Court, D.L.; Waugh, D.S.; Ji, X. Intermediate States of Ribonuclease III in Complex with Double-Stranded RNA. Structure 2005, 13, 1435–1442.
- Campbell, F.E.; Cassano, A.G.; Anderson, V.E.; Harris, M.E. Pre-steady-state and stopped-flow fluorescence analysis of Escherichia coli ribonuclease III: Insights into mechanism and conformational changes associated with binding and catalysis. J. Mol. Biol. 2002, 317, 21–40.
- Pertzev, A.V.; Nicholson, A.W. Characterization of RNA sequence determinants and antideterminants of processing reactivity for a minimal substrate of Escherichia coli ribonuclease III. Nucleic Acids Res. 2006, 34, 3708–3721.
- Dunn, J.J.; Studier, F.W.; Gottesman, M. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J. Mol. Biol. 1983, 166, 477–535.
- Panayotatos, N.; Truong, K. Cleavage within an RNase III site can control mRNA stability and protein synthesis in vivo. Nucleic Acids Res. 1985, 13, 2227–2240.
- Nicholson, A.W. Ribonuclease III mechanisms of double-stranded RNA cleavage. Wiley Interdiscip. Rev. RNA 2014, 5, 31–48.
- Altuvia, Y.; Bar, A.; Reiss, N.; Karavani, E.; Argaman, L.; Margalit, H. In vivo cleavage rules and target repertoire of RNase III in Escherichia coli. Nucleic Acids Res. 2018, 46, 10380–10394.
- Lybecker, M.; Zimmermann, B.; Bilusic, I.; Tukhtubaeva, N.; Schroeder, R. The double-stranded transcriptome of Escherichia coli. Proc. Natl. Acad. Sci. USA 2014, 111, 3134–3139.
- Thomason, M.K.; Bischler, T.; Eisenbart, S.K.; Forstner, K.U.; Zhang, A.; Herbig, A.; Nieselt, K.; Sharma, C.M.; Storz, G. Global transcriptional start site mapping using differential RNA sequencing reveals novel antisense RNAs in Escherichia coli. J. Bacteriol. 2015, 197, 18–28.
- Gordon, G.C.; Cameron, J.C.; Pfleger, B.F. RNA Sequencing Identifies New RNase III Cleavage Sites in Escherichia coli and Reveals Increased Regulation of mRNA. mBio 2017, 8.
- Gatewood, M.L.; Bralley, P.; Weil, M.R.; Jones, G.H. RNA-Seq and RNA immunoprecipitation analyses of the transcriptome of Streptomyces coelicolor identify substrates for RNase III. J. Bacteriol. 2012, 194, 2228–2237.
- Lioliou, E.; Sharma, C.M.; Caldelari, I.; Helfer, A.C.; Fechter, P.; Vandenesch, F.; Vogel, J.; Romby, P. Global regulatory functions of the Staphylococcus aureus endoribonuclease III in gene expression. PLoS Genet. 2012, 8.
- Gordon, G.C.; Cameron, J.C.; Pfleger, B.F. Distinct and redundant functions of three homologs of RNase III in the cyanobacterium Synechococcus sp. strain PCC 7002. Nucleic Acids Res. 2018, 46, 1984–1997.
- Spanka, D.-T.; Reuscher, C.M.; Klug, G. Impact of PNPase on the transcriptome of Rhodobacter sphaeroides and its cooperation with RNase III and RNase E. BMC Genom. 2021, 22, 106.
- Garza-Sánchez, F.; Schaub, R.E.; Janssen, B.D.; Hayes, C.S. tmRNA regulates synthesis of the ArfA ribosome rescue factor. Mol. Microbiol. 2011, 80, 1204–1219.
- Afonyushkin, T.; Vecerek, B.; Moll, I.; Blasi, U.; Kaberdin, V.R. Both RNase E and RNase III control the stability of sodB mRNA upon translational inhibition by the small regulatory RNA RyhB. Nucleic Acids Res 2005, 33, 1678–1689.
- Aiso, T.; Kamiya, S.; Yonezawa, H.; Gamou, S. Overexpression of an antisense RNA, ArrS, increases the acid resistance of Escherichia coli. Microbiology (Reading) 2014, 160, 954–961.
- Kavalchuk, K.; Madhusudan, S.; Schnetz, K. RNase III initiates rapid degradation of proU mRNA upon hypo-osmotic stress in Escherichia coli. RNA Biol. 2012, 9, 98–109.
- Sim, M.; Lim, B.; Sim, S.H.; Kim, D.; Jung, E.; Lee, Y.; Lee, K. Two tandem RNase III cleavage sites determine betT mRNA stability in response to osmotic stress in Escherichia coli. PLoS ONE 2014, 9.
- Lim, B.; Lee, K. Stability of the Osmoregulated Promoter-Derived proP mRNA Is Posttranscriptionally Regulated by RNase III in Escherichia coli. J. Bacteriol. 2015, 197, 1297–1305.
- Apirion, D.; Watson, N. Ribonuclease III is involved in motility of Escherichia coli. J. Bacteriol. 1978, 133, 1543–1545.
- Song, W.; Kim, Y.-H.; Sim, S.-H.; Hwang, S.; Lee, J.-H.; Lee, Y.; Bae, J.; Hwang, J.; Lee, K. Antibiotic stress-induced modulation of the endoribonucleolytic activity of RNase III and RNase G confers resistance to aminoglycoside antibiotics in Escherichia coli. Nucleic Acids Res. 2014, 42, 4669–4681.
- Wencker, F.D.R.; Marincola, G.; Schoenfelder, S.M.K.; Maaß, S.; Becher, D.; Ziebuhr, W. Another layer of complexity in Staphylococcus aureus methionine biosynthesis control: Unusual RNase III-driven T-box riboswitch cleavage determines met operon mRNA stability and decay. Nucleic Acids Res. 2021, 49, 2192–2212.
- Apura, P.; de Lorenzo, V.; Arraiano, C.M.; Martínez-García, E.; Viegas, S.C. Ribonucleases control distinct traits of Pseudomonas putida lifestyle. Environ. Microbiol. 2021, 23, 174–189.
- Salze, M.; Muller, C.; Bernay, B.; Hartke, A.; Clamens, T.; Lesouhaitier, O.; Rincé, A. Study of key RNA metabolism proteins in Enterococcus faecalis. RNA Biol. 2020, 17, 794–804.
- Wang, L.; Qiao, M.; Meng, Q.; Qiao, J.; Wu, Y.; Guo, J.; Wang, X.; Li, J.; Zhang, X.; Cai, X. Listeria monocytogenes in RNase III rncS. Kafkas Universitesi Veteriner Fakultesi Dergisi 2019.
- Svensson, S.L.; Sharma, C.M. An RNase III processed, antisense RNA pair regulates a Campylobacter jejuni colonization factor. bioRxiv 2021.
- Allen, A.G.; Morgans, S.; Smith, E.; Aron, M.M.; Jancovich, J.K. The Ambystoma tigrinum virus (ATV) RNase III gene can modulate host PKR activation and interferon production. Virology 2017, 511, 300–308.
- Xue, S.; Calvin, K.; Li, H. RNA Recognition and Cleavage by a Splicing Endonuclease. Science 2006, 312, 906–910.
- Thompson, L.D.; Daniels, C.J. Recognition of exon-intron boundaries by the Halobacterium volcanii tRNA intron endonuclease. J. Biol. Chem. 1990, 265, 18104–18111.
- Huntzinger, E.; Boisset, S.; Saveanu, C.; Benito, Y.; Geissmann, T.; Namane, A.; Lina, G.; Etienne, J.; Ehresmann, B.; Ehresmann, C.; et al. Staphylococcus aureus RNAIII and the endoribonuclease III coordinately regulate spa gene expression. EMBO J. 2005, 24, 824–835.
- Snow, S.; Bacon, E.; Bergeron, J.; Katzman, D.; Wilhelm, A.; Lewis, O.; Syangtan, D.; Calkins, A.; Archambault, L.; Anacker, M.L.; et al. Transcript decay mediated by RNase III in Borrelia burgdorferi. Biochem. Biophys. Res. Commun. 2020, 529, 386–391.
- Durand, S.; Gilet, L.; Condon, C. The essential function of B. subtilis RNase III is to silence foreign toxin genes. PLoS Genet. 2012, 8.
- Wang, W.; Bechhofer, D.H. Bacillus subtilis RNase III gene: Cloning, function of the gene in Escherichia coli, and construction of Bacillus subtilis strains with altered rnc loci. J. Bacteriol. 1997, 179, 7379–7385.
- Mitra, S.; Bechhofer, D.H. Substrate specificity of an RNase III-like activity from Bacillus subtilis. J. Biol. Chem. 1994, 269, 31450–31456.
- Redko, Y.; Bechhofer, D.H.; Condon, C. Mini-III, an unusual member of the RNase III family of enzymes, catalyses 23S ribosomal RNA maturation in B. subtilis. Mol. Microbiol. 2008, 68, 1096–1106.
- Rauhut, R.; Andreas, J.; Conrad, C.; Klug, G. Identification and Analysis of the rnc Gene for RNase III in Rhodobacter Capsulatus. Nucleic Acids Res. 1996, 24, 1246–1251.
- Conrad, C.; Rauhut, R.; Klug, G. Different cleavage specificities of RNases III from Rhodobacter capsulatus and Escherichia coli. Nucleic Acids Res. 1998, 26, 4446–4453.
- Xu, H.-P.; Riggs, M.; Rodgers, L.; Wigler, M. A gene from S. pombe with homology to E. coli RNAse III blocks conjugation and sporulation when overexpressed in wild type cells. Nucleic Acids Res. 1990, 18, 5304.
- Rotondo, G.; Gillespie, M.; Frendewey, D. Rescue of the fission yeast snRNA synthesis mutant snm1 by overexpression of the double-strand-specific Pac1 ribonuclease. Molec. Gen. Genet. 1995, 247, 698–708.
- Iino, Y.; Sugimoto, A.; Yamamoto, M. S. pombe pac1+, whose overexpression inhibits sexual development, encodes a ribonuclease III-like RNase. EMBO J. 1991, 10, 221–226.
- Elela, S.A.; Igel, H.; Ares, M., Jr. RNase III Cleaves Eukaryotic Preribosomal RNA at a U3 snoRNP-Dependent Site. Cell 1996, 85, 115–124.
- Rotondo, G.; Frendewey, D. Purification and Characterization of the Pac1 Ribonuclease of Schizosaccharomyces Pombe. Nucleic Acids Res. 1996, 24, 2377–2386.
- Filippov, V.; Solovyev, V.; Filippova, M.; Gill, S.S. A novel type of RNase III family proteins in eukaryotes. Gene 2000, 245, 213–221.
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001, 409, 363–366.
- Hammond, S.M. An overview of microRNAs. Adv. Drug Deliv. Rev. 2015, 87, 3–14.
- Carnes, J.; Soares, C.Z.; Wickham, C.; Stuart, K. Endonuclease Associations with Three Distinct Editosomes in Trypanosoma brucei. J. Biol. Chem. 2011, 286, 19320–19330.
- Madina, B.R.; Kuppan, G.; Vashisht, A.A.; Liang, Y.-H.; Downey, K.M.; Wohlschlegel, J.A.; Ji, X.; Sze, S.-H.; Sacchettini, J.C.; Read, L.K.; et al. Guide RNA biogenesis involves a novel RNase III family endoribonuclease in Trypanosoma brucei. RNA 2011, 17, 1821–1830.
- Watkins, K.P.; Kroeger, T.S.; Cooke, A.M.; Williams-Carrier, R.E.; Friso, G.; Belcher, S.E.; van Wijk, K.J.; Barkan, A. A Ribonuclease III Domain Protein Functions in Group II Intron Splicing in Maize Chloroplasts. Plant Cell 2007, 19, 2606–2623.
- Comella, P.; Pontvianne, F.; Lahmy, S.; Vignols, F.; Barbezier, N.; DeBures, A.; Jobet, E.; Brugidou, E.; Echeverria, M.; Sáez-Vásquez, J. Characterization of a ribonuclease III-like protein required for cleavage of the pre-rRNA in the 3′ETS in Arabidopsis. Nucleic Acids Res. 2007, 36, 1163–1175.
- Shamandi, N.; Zytnicki, M.; Charbonnel, C.; Elvira-Matelot, E.; Bochnakian, A.; Comella, P.; Mallory, A.C.; Lepère, G.; Sáez-Vásquez, J.; Vaucheret, H. Plants Encode a General siRNA Suppressor That Is Induced and Suppressed by Viruses. PLoS Biol. 2015, 13.
- Hotto, A.M.; Castandet, B.; Gilet, L.; Higdon, A.; Condon, C.; Stern, D.B. Arabidopsis Chloroplast Mini-Ribonuclease III Participates in rRNA Maturation and Intron Recycling. Plant Cell 2015, 27, 724–740.
More
Information
Subjects:
Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
27 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




