
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Beatriz Sales Bandarra | + 1938 word(s) | 1938 | 2021-10-27 05:01:50 | | | |
| 2 | Catherine Yang | -2 word(s) | 1936 | 2021-12-24 04:15:32 | | |
Video Upload Options
Incineration bottom ash (IBA) is the main residue from municipal solid waste (MSW) incineration and refers to the incombustible materials that remain in the furnace after combustion. IBA is a very heterogeneous material, comprising irregularly shaped particles and a wide particle size distribution. This material is a complex inorganic mixture generally composed of melt products, minerals, metallic compounds, ceramics, and glass [1]. The classification and the management practices of IBA differ worldwide and, particularly, among the EU Member States. However, different applications have been studied for this material.
1. Production
Municipal solid waste (MSW) corresponds to household waste or similar waste, which can be processed through incineration. MSW incineration process allows the combustion of waste with energy recovery (waste-to-energy). Incineration bottom ash (IBA) is the main solid output from the incineration process, accounting for about 80 wt.% of all incineration residues [1]. IBA is highly produced worldwide and, particularly, in Europe [2][3]. Generally, 1 t of MSW incinerated produces 150-250 kg of IBA [4]. In the EU-28, Switzerland and Norway there are 465 operational MSW incineration plants that burn around 90 Mt/year of MSW and industrial waste of similar composition. This results in around 18 Mt/year of IBA, which accounts for nearly 20 wt% of the annual incinerated waste [5]. Table 1 summarizes the number of incinerators, their capacity, and IBA generated in the EU-28, Switzerland and Norway in 2020.
Table 1. Number of incinerators, capacity, and IBA generated in the EU-28, Switzerland and Norway [5][6].
|
Country |
Number of incinerators |
Capacity (Mt/year) |
IBA produced (Mt/year) |
Country |
Number of incinerators |
Capacity (Mt/year) |
IBA produced (Mt/year) |
|
Austria |
11 |
2.6 |
0.53 |
Lithuania |
1 |
0.28 |
0.075 |
|
Belgium |
15 |
3.3 |
0.47 |
Luxembourg |
1 |
0.17 |
0.028 |
|
Czech Republic |
4 |
0.65 |
0.2 |
Poland |
6 |
0.97 |
0.21 |
|
Denmark |
24 |
3.7 |
0.6 |
Portugal |
4 |
1.2 |
0.24 |
|
Estonia |
1 |
0.25 |
0.058 |
Spain |
12 |
2.9 |
0.474 |
|
Finland |
9 |
1.6 |
0.3 |
Slovakia |
2 |
0.29 |
0.062 |
|
France |
126 |
14.7 |
2.9 |
Sweden |
34 |
5.4 |
0.99 |
|
Germany |
68 |
19.8 |
4.8 |
Netherlands |
12 |
7.6 |
1.9 |
|
Hungary |
1 |
0.42 |
0.12 |
United Kingdom |
45 |
12 |
1.5 |
|
Ireland |
2 |
0.8 |
0.14 |
Norway |
18 |
1.8 |
0.25 |
|
Italy |
39 |
6.1 |
1.03 |
Switzerland |
30 |
3.7 |
0.82 |
2. Characterization
The properties of IBA depend on the composition of the MSW feedstock, the combustion technology, and the operational conditions (e.g., incineration temperature) [7]. The moisture content in IBA represents from 7% to 30%, depending not only on the operational processes conditions but also on the post-combustion treatments and storage methods [8][9][10][11][12]. IBA is a very heterogeneous material and contains irregularly shaped particles with a porous microstructure. The average bulk density has been reported as 0.95–1.8 g/cm3 and the average specific gravity as 1.1–2.7 [10][12][13][14]. IBA is a complex (mainly) inorganic mixture composed of melted products, minerals, metallic compounds, ceramics, and glass [1]. In Europe, IBA is generally composed of the material fractions presented in Figure 1.
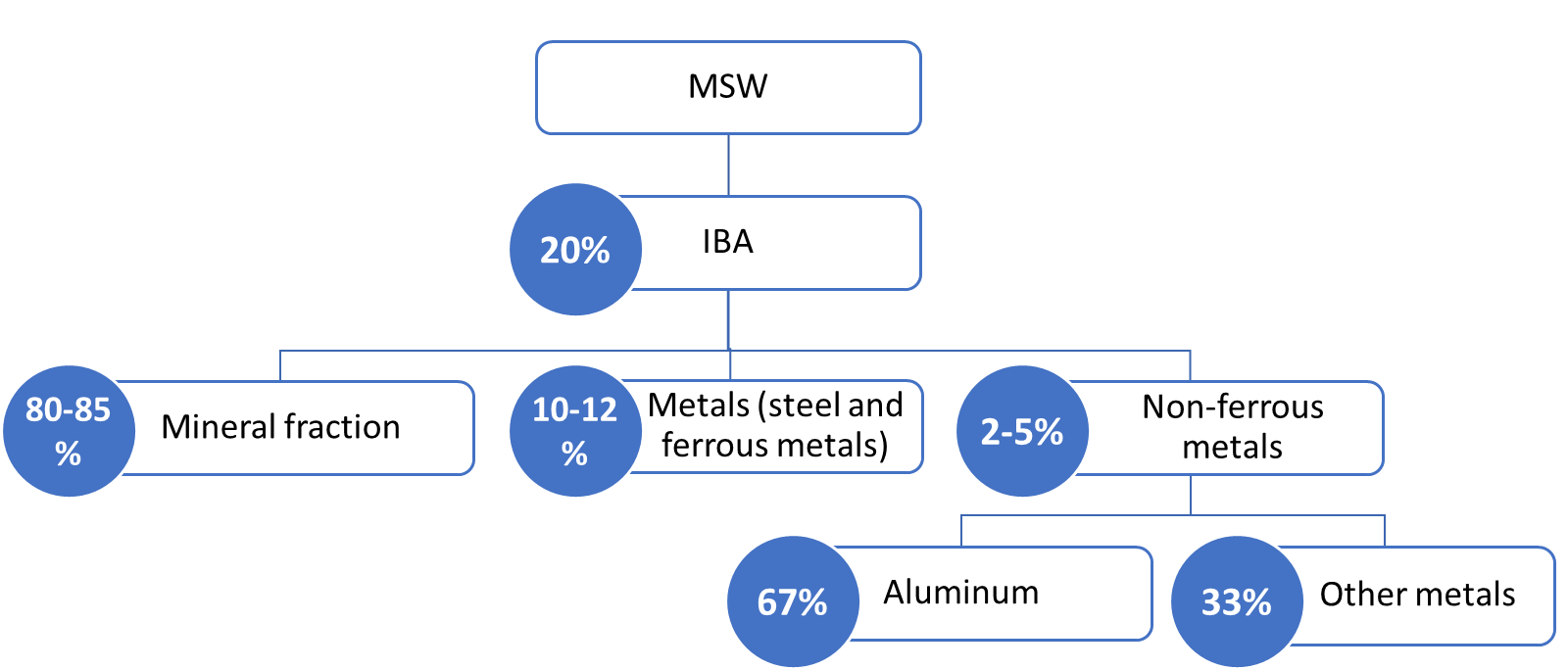
Figure 1 - Fractions of IBA in Europe, according to CEWEP (Confederation of European Waste-to-Energy Plants) [15]. Figure from Bandarra et al. (2021) [6].
In general, the particle size distribution of IBA covers a broad range, from a few μm to various cm [16]. According to Dou et al. [2], the main fraction of IBA has a particle size between 0.02 mm and 10 mm, accounting for 60-90 wt.%. Regarding the other fractions, 5 to 15% may contain a particle size below 0.02 mm, while < 30% can be higher than 10 mm. The larger particles normally include construction-type materials, pieces of glass, and ferrous and non-ferrous metals. While IBA finest fraction contains most of the soluble salts and the potentially leachable heavy metal(loid)s, the coarsest fractions mainly consist of synthetic ceramics (tiles, bricks, concrete blocks) and container glass. However, it should be noted that the particle size distribution depends on the technology used (grates or fluidized bed) and on the feed to the combustion chamber (MSW or refuse-derived fuel). The chemical composition may differ depending on the MSW input and the combustion conditions.
The main elements of IBA expressed as oxides are SiO2, CaO, Fe2O3, Na2O, Al2O3, P2O5, MgO, K2O, TiO2, and SO3 (Table 2). Typically, fresh IBA is alkaline with a pH between 10 and 13 [9][17]. However, pH can vary as a function of the particle size fractions and composition: finer fractions tend to have a higher initial content of portlandite, and in the coarser fractions portlandite is scarcer and undergo carbonation faster [18].
Potentially toxic metals such as Cd, Cr, Cu, Ni, Pb, and Zn may be found in IBA (Table 2) due to their presence in MSW, and their leaching patterns may be linked to the presence of chlorides [19][20]. Thus, there are some environmental concerns regarding IBA [21][22] related to the potential contamination of vulnerable recipient compartments, such as water bodies and groundwaters, ultimately affecting the inhabiting biological communities [23][24][25][26]. However, as the chemical composition of IBA may vary significantly as a function of the particle size distribution [9][18][21][27], higher concentrations of chlorides and potentially toxic metals (e.g., Cr, Cu, Hg, Mo, Pb, Sb, Zn) have been detected for smaller IBA particles, namely fractions under 4 mm [1][17][27][28][29][30].
Furthermore, most di- and trivalent potentially toxic metal(loid)s are pH-dependent, and under natural weathering conditions, the leaching potential can decrease considerably due to pH decreasing [18][31]. Indeed, some of these metals can be retained in the neoformed mineral phases (calcite, ettringite, aluminosilicates, metal oxides, etc.). Therefore, the release of heavy metal(loid)s decreases during the aging period, and after 2-3 months in the open conditions, IBA may be classified as a non-hazardous material [31].
Table 2. Chemical composition of IBA from MSW based on the literature a.
|
Constituents |
Concentration (wt %) |
Constituents |
Concentration (mg/kg) |
Constituents |
Concentration (mg/kg) |
|
Oxides |
Elements |
Elements |
|||
|
SiO2 |
22 - 64.84 |
Ag |
0.28 - 38 |
Sb |
10 - 86 |
|
CaO |
10.45 - 42.9 |
As |
0.12 - 189 |
Sn |
2 - 960 |
|
Al2O3 |
5 - 31.31 |
Ba |
400 - 3920 |
Sr |
85 - 1000 |
|
Fe2O3 |
4. 64 - 15.13 |
Cd |
0.3 - 146 |
V |
20 - 122 |
|
MgO |
1.18 - 4.62 |
Co |
6 - 350 |
Zn |
613 - 7770 |
|
Na2O |
1.53 -7.78 |
Cr |
23 - 3170 |
Pb |
98 - 13700 |
|
K2O |
0.83 - 1.66 |
Cu |
190 - 12000 |
||
|
P2O5 |
0.55 - 5.5 |
Hg |
0.02 - 7.75 |
||
|
TiO2 |
0.5 - 2.17 |
Mn |
465 - 3408 |
||
|
SO3 |
0.57-2.18 |
Mo |
2.5 - 276 |
||
|
Clb |
0.18 - 7 |
Ni |
7 - 4280 |
a [7][29][32][33][34][35][36][37][38][39][40][41][42][43][44]
b Chlorine content expressed as an element.
3. Management
The ferrous materials that are generally recovered from IBA are an absolute entry of non-hazardous waste in the European List of Waste (LoW, revised by EU Decision 2014/955/EU) with the code 19 01 02. Although generally classified as non-hazardous [32][45][46], the remaining material of bottom ashes appears as a mirror entry in the LoW (codes 19 01 11* and 19 01 12). This means that its classification relies on the evaluation of waste properties that render it hazardous in line with Regulation (EU) No 1357/2014, as well as on the assessment of persistent organic pollutants (POP) according to Regulation (EU) 2019/1021. As a result, there are different approaches for the management of IBA worldwide and, particularly, among the EU Member States [2][5]. According to Blasenbauer et al. (2020)[5], 16 out of 22 EU countries, plus Norway, and Switzerland allow the utilization of mineral IBA outside landfills. However, only 11 of them use it, at a rate varying from 20 to 100 wt%.
Given the large amounts of IBA produced, efforts have been made to valorize this material considering different applications instead of disposing of it in landfills. Indeed, IBA has the potential for the recovery of metals and minerals, contributing to decreasing the exploitation of natural resources and it has been largely recycled in different countries. Ferrous and non-ferrous metals are usually recovered through magnetic separation and Eddy current separation techniques, respectively [12][47]. Nearly 80% of the metals can be recovered from IBA, and metals such as aluminum, copper, steel, and zinc are frequently separated and applied as secondary raw materials [3]. The remaining ashes are generally landfilled (frequently after a solidification process with Portland cement) or used in different applications according to the policy of each country. Indeed, the rate of usage varies between 100 wt.% and 0 wt.% (i.e., 100 wt% disposed of in landfills) and the related regulations diverge among countries, particularly within the EU [2][3][5][16][26][33].
Before reuse, different treatments may be applied to reduce the mobility of potentially hazardous constituents from IBA. Depending on the intended IBA application, these treatments may include natural weathering, washing, heat treatment, particle density-based separation, and stabilization with the addition of hydraulic binders [26][48]. Natural weathering is the most used treatment. In this case, IBA is stored outdoors exposed to ambient conditions for 6 to 20 weeks to undergo an aging/weathering process [3][49]. This results in the neoformation and hydration of the mineral phases involving carbonation and oxidation reactions, which leads to a pH reduction into the range 8-10 [31][49]. The reactions of hydration originate mineral species that can encapsulate some potentially toxic metals, leading to an enhanced leaching behavior [26][50][51][52][53][54][55][56].
There are several potential applications of the mineral fraction of IBA. The main attraction of IBA is its particle size distribution and its composition rich in glass, ceramics, stone, brick, concrete, ash, and melting products. Indeed, the main application of the weathered bottom ash is found in the civil and building engineering field as a secondary aggregate material. The replacement of aggregates has been an appealing use for IBA due to its geotechnical properties [2]. Many European countries apply IBA as a secondary raw material to replace natural materials (e.g., gravel and sand). Belgium, Denmark, France, Germany, the Netherlands, the United Kingdom, Portugal, and Spain use IBA in road construction. IBA has been also applied in acoustic barriers for roads in Germany and the Netherlands [3]. Other common functions are the use in cement production [10][57], as aggregate for concrete [3][10][41][58], embankments [59], and as landfill cover [2][3]. Other constructive character applications are focused on the sintering of the IBA at high temperatures (above 1000 °C) to obtain ceramics [60], glass-ceramics [37], bricks [61], and tiles [62]. In the chemical engineering field, studies have been carried out for its use as an adsorbent for wastewater treatment processes and gas separation and purification through capturing hazardous elements [63][64]. It is also assessed the use of IBA in co-disposal and biogas production in landfills, as well as to protect wastes from pests and to avoid scattering of lightweight residues [65]. Finally, the alkali activation of IBA to produce alkali-activated binders is presented as a new alternative application [66].
4. Prospects for utilization
Proper and environmentally sound utilization of anthropogenic resources like IBA may contribute to the circular economy, decreasing the consumption of natural resources. Moreover, the utilization of IBA also allows to reduce the amount of waste landfilled and related impacts, pollution of groundwater and soil, odor emission, and loss of resources potentially recyclable [26][32][67][68]. Additionally, diverting waste from landfills provides economic benefits, since landfill costs, taxes, and costs of mining raw materials are prevented [69].
However, the classification of IBA as a “waste” leads to constraints in its management. Particularly, the classification as “mirror entry” in the LoW results itself in major differences in IBA management among countries. In this context, the proper assessment of the hazardous property HP 14 “ecotoxic”, related to potential environmental impacts, is of major importance to classify mirror entries since it is responsible for most of the hazardous entries in the LoW [67][70]. Currently, there is no consensus in the scientific community on the approach that should be followed, and there are different proposals in the literature [71][72][73][74][75][76][77][78][79][80]. However, it should be noted that a potential classification as “hazardous waste” is not necessarily directly linked to the environmental risks associated with its use as a product and should not automatically lead to valorization barriers. Raw waste and products thereof may not have the same environmental impact.
In fact, environmental contamination is one of the main concerns in using new materials. the technical feasibility of using IBA in different applications, its environmental performance is still being discussed and is not broadly known. The main concern in the IBA applications is the potential leaching of heavy metalloids, chlorides, and sulphates [81]. For example, IBA utilization in road construction is more beneficial than landfilling, but the benefits may not be verified for high amounts of elements released if the leaching behavior of IBA is not properly controlled [26]. Natural weathering/aging prior to IBA utilization is referred to as a form of enhancing the environmental performance since that process originates a more stable material.
Thus, it is foreseeable that more studies will be focused on this waste, particularly regarding finding alternatives for its application while ensuring environmental protection, pursuing a circular economy.
References
- Chimenos, J.M.; Segarra, M.; Fernández, M.A.; Espiell, F; Characterization of the bottom ash in municipal solid waste incinerator. Journal of Hazardous Materials 1999, 64, 211-222, 10.1016/s0304-3894(98)00246-5.
- Dou, X.; Ren, F.; Nguyen, M.Q.; Ahamed, A.; Yin, K.; Chan, W.P.; Chang, V.W.C.; Review of MSWI bottom ash utilization from perspectives of collective characterization, treatment and existing application. Renewable and Sustainable Energy Reviews 2017, 79, 24-38, 10.1016/j.rser.2017.05.044.
- Bottom Ash Factsheet . CEWEP - Confederation of European Waste-to-Energy Plants. Retrieved 2021-12-24
- Hyks, J.; Hjelmar, O.. Utilisation of Incineration Bottom Ash (IBA) from Waste Incineration - Prospects and Limits. In Removal, Treatment and Utilisation of Waste Incineration Bottom Ash; Holm, O., Thomé-Kozmiensky, E., Eds.; Thomé-Kozmiensky Verlag GmbH: Neuruppin, 2018; pp. 11–23.
- Blasenbauer, D.; Huber, F.; Lederer, J.; Quina, M.J.; Blanc-Biscarat, D.; Bogush, A.; Bontempi, E.; Blondeau, J.; Chimenos, J.M.; Dahlbo, H.; et al.et al. Legal situation and current practice of waste incineration bottom ash utilisation in Europe. Waste Management 2020, 102, 868-883, 10.1016/j.wasman.2019.11.031.
- Bandarra, B.S.; Pereira, J.L.; Martins, R.C.; Maldonado-Alameda, A.; Chimenos, J.M.; Quina, M.J.; Opportunities and Barriers for Valorizing Waste Incineration Bottom Ash: Iberian Countries as a Case Study. Applied Sciences 2021, 11, 9690, 10.3390/app11209690.
- Rendek, E.; Ducom, G.; Germain, P.; Influence of waste input and combustion technology on MSWI bottom ash quality. Waste Management 2007, 27, 1403-1407, 10.1016/j.wasman.2007.03.016.
- Alhassan, H.M.; Tanko, A.M. Characterization of solid waste incinerator bottom ash and the potential for its use Characterization of Solid Waste Incinerator Bottom Ash and the Potential for its Use. International Journal of Engineering Research and Applications 2012, 2, 516–522.
- Yin, K.; Chan, W.P.; Dou, X.; Ren, F.; Wei-Chung Chang, V.; Cr, Cu, Hg and Ni release from incineration bottom ash during utilization in land reclamation – based on lab-scale batch and column leaching experiments and a modeling study. Chemosphere 2018, 197, 741-748, 10.1016/j.chemosphere.2018.01.107.
- Joseph, A.M.; Snellings, R.; Van den Heede, P.; Matthys, S.; De Belie, N.; The Use of Municipal Solid Waste Incineration Ash in Various Building Materials: A Belgian Point of View. Materials 2018, 11, 141, 10.3390/ma11010141.
- Seniunaite, J.; Vasarevicius, S.; Leaching of Copper, Lead and Zinc from Municipal Solid Waste Incineration Bottom Ash. Energy Procedia 2017, 113, 442-449, 10.1016/j.egypro.2017.04.036.
- Wiles, C.C.; Municipal solid waste combustion ash: State-of-the-knowledge. Journal of Hazardous Materials 1996, 47, 325-344, 10.1016/0304-3894(95)00120-4.
- Lynn, C.J.; Ghataora, G.S.; Dhir OBE, R.K.; Municipal incinerated bottom ash (MIBA) characteristics and potential for use in road pavements. International Journal of Pavement Research and Technology 2017, 10, 185-201, 10.1016/j.ijprt.2016.12.003.
- Šyc, M.; Simon, F.G.; Hykš, J.; Braga, R.; Biganzoli, L.; Costa, G.; Funari, V.; Grosso, M.; Metal recovery from incineration bottom ash: State-of-the-art and recent developments. Journal of Hazardous Materials 2020, 393, 122433, 10.1016/j.jhazmat.2020.122433.
- What is Waste-to-Energy - Incineration? . CEWEP - Confederation of European Waste-to-Energy Plants. Retrieved 2021-12-24
- Huber, F.; Blasenbauer, D.; Aschenbrenner, P.; Fellner, J.; Complete determination of the material composition of municipal solid waste incineration bottom ash. Waste Management 2020, 102, 677-685, 10.1016/j.wasman.2019.11.036.
- Luo, H.; Cheng, Y.; He, D.; Yang, E.H.; Review of leaching behavior of municipal solid waste incineration (MSWI) ash. Science of The Total Environment 2019, 668, 90-103, 10.1016/j.scitotenv.2019.03.004.
- Chimenos, J.M.; Fernández, A.I.; Miralles, L.; Segarra, M.; Espiell, F.; Short-term natural weathering of MSWI bottom ash as a function of particle size. Waste Management 2003, 23, 887-895, 10.1016/s0956-053x(03)00074-6.
- Van Der Sloot, H.A.; Kosson, D.S.; Hjelmar, O.; Characteristics, treatment and utilization of residues from municipal waste incineration. Waste Management 2001, 21, 753-765, 10.1016/s0956-053x(01)00009-5.
- Weibel, G.; Eggenberger, U.; Kulik, D.A.; Hummel, W.; Schlumberger, S.; Klink, W.; Fisch, M.; Mäder, U.K.; Extraction of heavy metals from MSWI fly ash using hydrochloric acid and sodium chloride solution. Waste Management 2018, 76, 457-471, 10.1016/j.wasman.2018.03.022.
- Crillesen, K.; Skaarup, J.; Bojsen., K. Management of Bottom Ash from WTE Plants - An overview of management options and treatment methods; Copenhagen, Denmark, 2006.
- del Valle-zermeño, R.; Chimenos, J.M.; Giró-paloma, J.; Formosa, J.; Use of weathered and fresh bottom ash mix layers as a subbase in road constructions: Environmental behavior enhancement by means of a retaining barrier. Chemosphere 2014, 117, 402-409, 10.1016/j.chemosphere.2014.07.095.
- Fuchs, B.; Track, C.; Lang, S.; Gimmler, H. Salt effects of processed municipal solid waste incinerator bottom ash on vegetation and underground water.Journal of Applied Botany and Food Quality. 1997, 71, 154–163.
- Shih, H. ching; Ma, H. wen; Life cycle risk assessment of bottom ash reuse. Journal of Hazardous Materials 2011, 190, 308-316, 10.1016/j.jhazmat.2011.03.053.
- Shih, H. ching; Ma, H. wen; Assessing the health risk of reuse of bottom ash in road paving. Chemosphere 2011, 82, 1556-1562, 10.1016/j.chemosphere.2010.11.061.
- Silva, R. V.; de Brito, J.; Lynn, C.J.; Dhir, R.K.; Environmental impacts of the use of bottom ashes from municipal solid waste incineration: A review. Resources, Conservation and Recycling 2019, 140, 23-35, 10.1016/j.resconrec.2018.09.011.
- Alam, Q.; Schollbach, K.; Hoek, C. Van; Laan, S. Van Der; Wolf, T. De; Brouwers, H.J.H.; In-depth mineralogical quantification of MSWI bottom ash phases and their association with potentially toxic elements. Waste Management 2019, 87, 1-12, 10.1016/j.wasman.2019.01.031.
- Chen, C.H.; Chiou, I.J.; Distribution of chloride ion in MSWI bottom ash and de-chlorination performance. Journal of Hazardous Materials 2007, 148, 346-352, 10.1016/j.jhazmat.2007.02.046.
- Tang, J.; Steenari, B.M.; Leaching optimization of municipal solid waste incineration ash for resource recovery: A case study of Cu, Zn, Pb and Cd. Waste Management 2016, 48, 315-322, 10.1016/j.wasman.2015.10.003.
- Xia, Y.; He, P.; Shao, L.; Zhang, H.; Metal distribution characteristic of MSWI bottom ash in view of metal recovery. Journal of Environmental Sciences 2017, 52, 178-189, 10.1016/j.jes.2016.04.016.
- Chimenos, J.M.; Fernández, A.I.; Nadal, R.; Espiell, F.; Short-term natural weathering of MSWI bottom ash. Journal of Hazardous Materials 2000, 79, 287-299, 10.1016/s0304-3894(00)00270-3.
- Monteiro, R.C.C.; Figueiredo, C.F.; Alendouro, M.S.; Ferro, M.C.; Davim, E.J.R.; Fernandes, M.H.V.; Characterization of MSWI bottom ashes towards utilization as glass raw material. Waste Management 2008, 28, 1119-1125, 10.1016/j.wasman.2007.05.004.
- Neuwahl, F.; Cusano, G.; Benavides, J.G.; Holbrook, S.; Serge, R. Best Available Techniques (BAT) Reference Document for Waste Incineration. Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention); Science for Policy report by the Joint Research Centre, European Commission, Luxembourg, 2019
- Rambaldi, E.; Esposito, L.; Andreola, F.; Barbieri, L.; Lancellotti, I.; Vassura, I.; The recycling of MSWI bottom ash in silicate based ceramic. Ceramics International 2010, 36, 2469-2476, 10.1016/j.ceramint.2010.08.005.
- Tang, P.; Florea, M.V.A.; Spiesz, P.; Brouwers, H.J.H.; Characteristics and application potential of municipal solid waste incineration (MSWI) bottom ashes from two waste-to-energy plants. Construction and Building Materials 2015, 83, 77-94, 10.1016/j.conbuildmat.2015.02.033.
- Zhang, Z.; Zhang, L.; Li, A.; Development of a sintering process for recycling oil shale fly ash and municipal solid waste incineration bottom ash into glass ceramic composite. Waste Management 2015, 38, 185-193, 10.1016/j.wasman.2014.12.028.
- Andreola, F.; Barbieri, L.; Hreglich, S.; Lancellotti, I.; Morselli, L.; Passarini, F.; Vassura, I.; Reuse of incinerator bottom and fly ashes to obtain glassy materials. Journal of Hazardous Materials 2008, 153, 1270-1274, 10.1016/j.jhazmat.2007.09.103.
- Ashraf, M.S.; Ghouleh, Z.; Shao, Y.; Production of eco-cement exclusively from municipal solid waste incineration residues. Resources, Conservation and Recycling 2019, 149, 332-342, 10.1016/j.resconrec.2019.06.018.
- Bayuseno, A.P.; Schmahl, W.W.; Understanding the chemical and mineralogical properties of the inorganic portion of MSWI bottom ash. Waste Management 2010, 30, 1509-1520, 10.1016/j.wasman.2010.03.010.
- Forteza, R.; Far, M.; Seguí, C.; Cerdá,; Characterization of bottom ash in municipal solid waste incinerators for its use in road base. Waste Management 2004, 24, 899-909, 10.1016/j.wasman.2004.07.004.
- Ginés, O.; Chimenos, J.M.; Vizcarro, A.; Formosa, J.; Rosell, J.R.; Combined use of MSWI bottom ash and fly ash as aggregate in concrete formulation: Environmental and mechanical considerations. Journal of Hazardous Materials 2009, 169, 643-650, 10.1016/j.jhazmat.2009.03.141.
- Huber, F.; Blasenbauer, D.; Aschenbrenner, P.; Fellner, J.; Chemical composition and leachability of differently sized material fractions of municipal solid waste incineration bottom ash. Waste Management 2019, 95, 593-603, 10.1016/j.wasman.2019.06.047.
- Hyks, J.; Astrup, T.; Christensen, T.H.; Leaching from MSWI bottom ash: Evaluation of non-equilibrium in column percolation experiments. Waste Management 2009, 29, 522-529, 10.1016/j.wasman.2008.06.011.
- Lindberg, D.; Molin, C.; Hupa, M.; Thermal treatment of solid residues from WtE units: A review. Waste Management 2015, 37, 82-94, 10.1016/j.wasman.2014.12.009.
- BIO by Deloitte. Study to assess the impacts of different classification approaches for hazard property “HP 14” on selected waste streams - Final report; Prepared for the European Commission (DG ENV), in collaboration with INERIS., 2015
- Stiernström, S.; Wik, O.; Bendz, D.; Evaluation of frameworks for ecotoxicological hazard classification of waste. Waste Management 2016, 58, 14-24, 10.1016/j.wasman.2016.08.030.
- Oehmig, W.N.; Roessler, J.G.; Zhang, J.; Townsend, T.G.; Effect of ferrous metal presence on lead leaching in municipal waste incineration bottom ashes. Journal of Hazardous Materials 2015, 283, 500-506, 10.1016/j.jhazmat.2014.09.040.
- Dhir, R.K.; De Brito, J.; Lynn, C.J.; Silva, R.V. Sustainable Construction Materials: Municipal Incinerator Bottom Ashes; Woodhead Publishing: Duxford, UK, 2018.
- Maldonado-Alameda, A.; Giro-Paloma, J.; Svobodova-Sedlackova, A.; Formosa, J.; Chimenos, J.M.; Municipal solid waste incineration bottom ash as alkali-activated cement precursor depending on particle size. Journal of Cleaner Production 2020, 242, 118443, 10.1016/j.jclepro.2019.118443.
- Baciocchi, R.; Costa, G.; Lategano, E.; Marini, C.; Polettini, A.; Pomi, R.; Postorino, P.; Rocca, S.; Accelerated carbonation of different size fractions of bottom ash from RDF incineration. Waste Management 2010, 30, 1310-1317, 10.1016/j.wasman.2009.11.027.
- Cornelis, G.; Gerven, T. Van; Vandecasteele, C.; Antimony leaching from uncarbonated and carbonated MSWI bottom ash. Journal of Hazardous Materials 2006, 137, 1284-1292, 10.1016/j.jhazmat.2006.04.048.
- Cornelis, G.; Gerven, T. Van; Vandecasteele, C.; Antimony leaching from MSWI bottom ash: Modelling of the effect of pH and carbonation. Waste Management 2012, 32, 278-286, 10.1016/j.wasman.2011.09.018.
- Shimaoka, T.; Zhang, R.; Watanabe, K.; Alterations of municipal solid waste incineration residues in a landfill. Waste Management 2007, 27, 1444-1451, 10.1016/j.wasman.2007.03.011.
- Wei, Y.; Saffarzadeh, A.; Shimaoka, T.; Zhao, C.; Peng, C.; Gao, J.; Geoenvironmental weathering/deterioration of landfilled MSWI-BA glass. Journal of Hazardous Materials 2014, 278, 610-619, 10.1016/j.jhazmat.2014.05.093.
- Wei, Y.; Shimaoka, T.; Saffarzadeh, A.; Takahashi, F.; Alteration of municipal solid waste incineration bottom ash focusing on the evolution of iron-rich constituents. Waste Management 2011, 31, 1992-2000, 10.1016/j.wasman.2011.04.021.
- Wei, Y.; Shimaoka, T.; Saffarzadeh, A.; Takahashi, F.; Mineralogical characterization of municipal solid waste incineration bottom ash with an emphasis on heavy metal-bearing phases. Journal of Hazardous Materials 2011, 187, 534-543, 10.1016/j.jhazmat.2011.01.070.
- Siddique, R.; Utilization of municipal solid waste (MSW) ash in cement and mortar. Resources, Conservation and Recycling 2010, 54, 1037-1047, 10.1016/j.resconrec.2010.05.002.
- Jurič, B.; Hanžič, L.; Ilić, R.; Samec, N.; Utilization of municipal solid waste bottom ash and recycled aggregate in concrete. Waste Management 2006, 26, 1436-1442, 10.1016/j.wasman.2005.10.016.
- Pecqueur, G.; Crignon, C.; Quénée, B. Behaviour of cement-treated MSWI bottom ash. Waste Management 2001, 229–233, doi:10.1016/S0713-2743(00)80065-3.
- Cheeseman, C.R.; Monteiro Da Rocha, S.; Sollars, C.; Bethanis, S.; Boccaccini, A.R.; Ceramic processing of incinerator bottom ash. Waste Management 2003, 23, 907-916, 10.1016/s0956-053x(03)00039-4.
- Andreola, F.; Barbieri, L.; Lancellotti, I.; Pozzi, P.; Recycling industrial waste in brick manufacture. Part 1.. Materiales de Construcción 2005, 55, 5-16, 10.3989/mc.2005.v55.i280.202.
- Barbieri, L.; Corradi, A.; Lancellotti, I.; Manfredini, T.; Use of municipal incinerator bottom ash as sintering promoter in industrial ceramics. Waste Management 2002, 22, 859-863, 10.1016/s0956-053x(02)00077-6.
- Shim, Y.; Kim, Y.; Kong, S.; Rhee, S.; Lee, W.; The adsorption characteristics of heavy metals by various particle sizes of MSWI bottom ash. Waste Management 2003, 23, 851-857, 10.1016/s0956-053x(02)00163-0.
- Wang, Y.; Huang, L.; Lau, R.; Conversion of municipal solid waste incineration bottom ash to sorbent material for pollutants removal from water. Journal of the Taiwan Institute of Chemical Engineers 2016, 60, 275-286, 10.1016/j.jtice.2015.10.013.
- Yao, J.; Kong, Q.; Li, W.; Zhu, H.; Shen, D.S.; Effect of leachate recirculation on the migration of copper and zinc in municipal solid waste and municipal solid waste incineration bottom ash co-disposed landfill. Journal of Material Cycles and Waste Management 2014, 16, 775-783, 10.1007/s10163-013-0217-7.
- Maldonado-Alameda, À.; Giro-Paloma, J.; Alfocea-Roig, A.; Formosa, J.; Chimenos, J.M.; Municipal Solid Waste Incineration Bottom Ash as Sole Precursor in the Alkali-Activated Binder Formulation. Applied Sciences 2020, 10, 4129, 10.3390/app10124129.
- Pandard, P.; Devillers, J.; Charissou, A.M.; Poulsen, V.; Jourdain, M.J.; Férard, J.F.; Grand, C.; Bispo; Selecting a battery of bioassays for ecotoxicological characterization of wastes. Science of The Total Environment 2006, 363, 114-125, 10.1016/j.scitotenv.2005.12.016.
- What is Waste-to-Energy - Incineration? . CEWEP - Confederation of European Waste-to-Energy Plants. Retrieved 2021-12-24
- Verbinnen, B.; Billen, P.; Van Caneghem, J.; Vandecasteele, C.; Recycling of MSWI Bottom Ash: A Review of Chemical Barriers, Engineering Applications and Treatment Technologies. Waste and Biomass Valorization 2017, 8, 1453-1466, 10.1007/s12649-016-9704-0.
- Hennebert, P.; Proposal of concentration limits for determining the hazard property HP 14 for waste using ecotoxicological tests. Waste Management 2018, 74, 74-85, 10.1016/j.wasman.2017.11.048.
- Klymko, T.; Dijkstra, J.J.; van Zomeren., A. Guidance document on hazard classification of MSWI bottom ash. ECN report; Petten, the Netherlands, 2017
- Wahlström, M.; Laine-Ylijok, J.; Wik, O.; Oberender, A.; Hjelmar, O. Hazardous waste classification: Amendments to the European Waste Classification regulation – what do they mean and what are the consequences? Report for the Nordic Council of Ministers.; Copenhagen, Denmark, 2016
- WRc. Assessment of Hazard Classification of UK IBA. Report for the January-June 2011 IBA dataset. Report for Environmental Services Association. WRc reference: UC8540.06.; 2012
- Hjelmar, O.; Van Der Sloot, H.A.; Van Zomeren, A. Hazard Property Classification of High Temperature Waste Materials. In Proceedings Sardinia 2013, Fourteenth InternationalWaste Management and Landfill Symposium, S. Margherita di Pula, Cagliari, Italy, 30 September–4 October 2013; CISA Publisher: Cagliari, Italy, 2013.
- Ferrari, B.; Radetski, C.M.; Veber, A.M.; Ferard, J.F.; Ecotoxicological assessment of solid wastes: A combined liquid- and solid-phase testing approach using a battery of bioassays and biomarkers. Environmental Toxicology and Chemistry 1999, 18, 1195–1202, 10.1897/1551-5028(1999)018<1195:eaoswa>2.3.co;2.
- Lapa, N.; Barbosa, R.; Morais, J.; Mendes, B.; Méhu, J.; Santos Oliveira, J.F.; Ecotoxicological assessment of leachates from MSWI bottom ashes. Waste Management 2002, 22, 583-593, 10.1016/s0956-053x(02)00009-0.
- Moser, H.; Römbke, J. Ecotoxicological characterization of waste - Results and experiences of a European ring test; Springer Ltd.: New York, USA, 2009; ISBN 9780387889580.
- Römbke, J.; Moser, T.; Moser, H.; Ecotoxicological characterisation of 12 incineration ashes using 6 laboratory tests. Waste Management 2009, 29, 2475-2482, 10.1016/j.wasman.2009.03.032.
- Pandard, P.; Römbke, J.; Proposal for a “Harmonized” strategy for the assessment of the HP 14 property. Integrated Environmental Assessment and Management 2013, 9, 665-672, 10.1002/ieam.1447.
- Ribé, V.; Nehrenheim, E.; Odlare, M.; Assessment of mobility and bioavailability of contaminants in MSW incineration ash with aquatic and terrestrial bioassays. Waste Management 2014, 34, 1871-1876, 10.1016/j.wasman.2013.12.024.
- Xuan, D.; Tang, P.; Poon, C.S.; Limitations and quality upgrading techniques for utilization of MSW incineration bottom ash in engineering applications – A review. Construction and Building Materials 2018, 190, 1091-1102, 10.1016/j.conbuildmat.2018.09.174.





