
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xunlei Kang | + 1690 word(s) | 1690 | 2021-12-14 06:45:23 | | | |
| 2 | Vicky Zhou | Meta information modification | 1690 | 2021-12-24 01:55:11 | | |
Video Upload Options
Acute myeloid leukemia (AML) is a heterogeneous disease associated with various alterations in T cell phenotype and function leading to an abnormal cell population, ultimately leading to immune exhaustion. However, restoration of T cell function allows for the execution of cytotoxic mechanisms against leukemic cells in AML patients. Therefore, long-term disease control, which requires multiple therapeutic approaches, includes those aimed at the re-establishment of cytotoxic T cell activity. AML treatments that harness the power of T lymphocytes against tumor cells have rapidly evolved over the last 3 to 5 years through various stages of preclinical and clinical development. These include tissue-infiltrated lymphocytes (TILs), bispecific antibodies, immune checkpoint inhibitors (ICIs), chimeric antigen receptor T (CAR-T) cell therapy, and tumor-specific T cell receptor gene-transduced T (TCR-T) cells.
1. Introduction
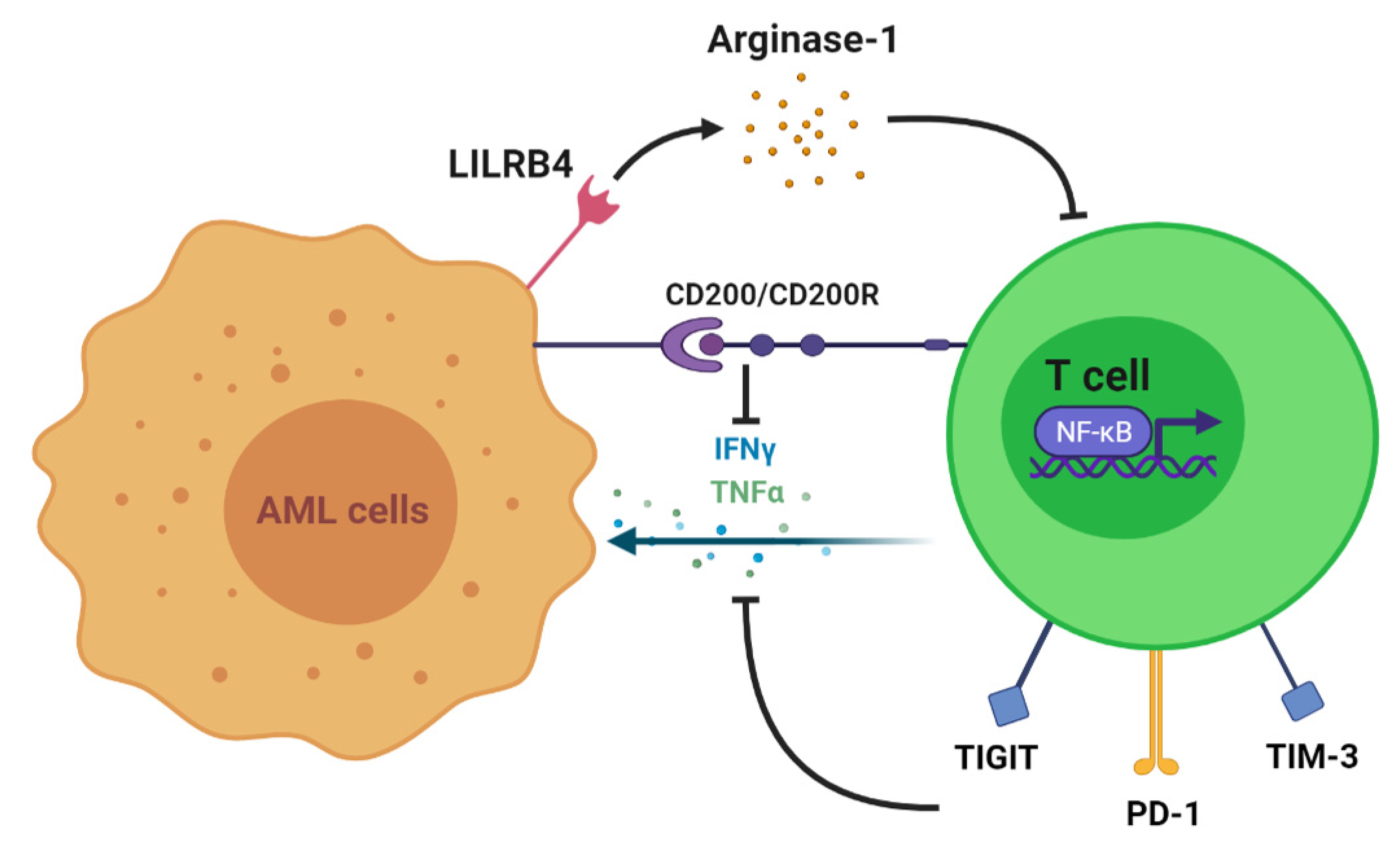
2. T Cell Alteration in AML
3. T Cell Immunotherapy
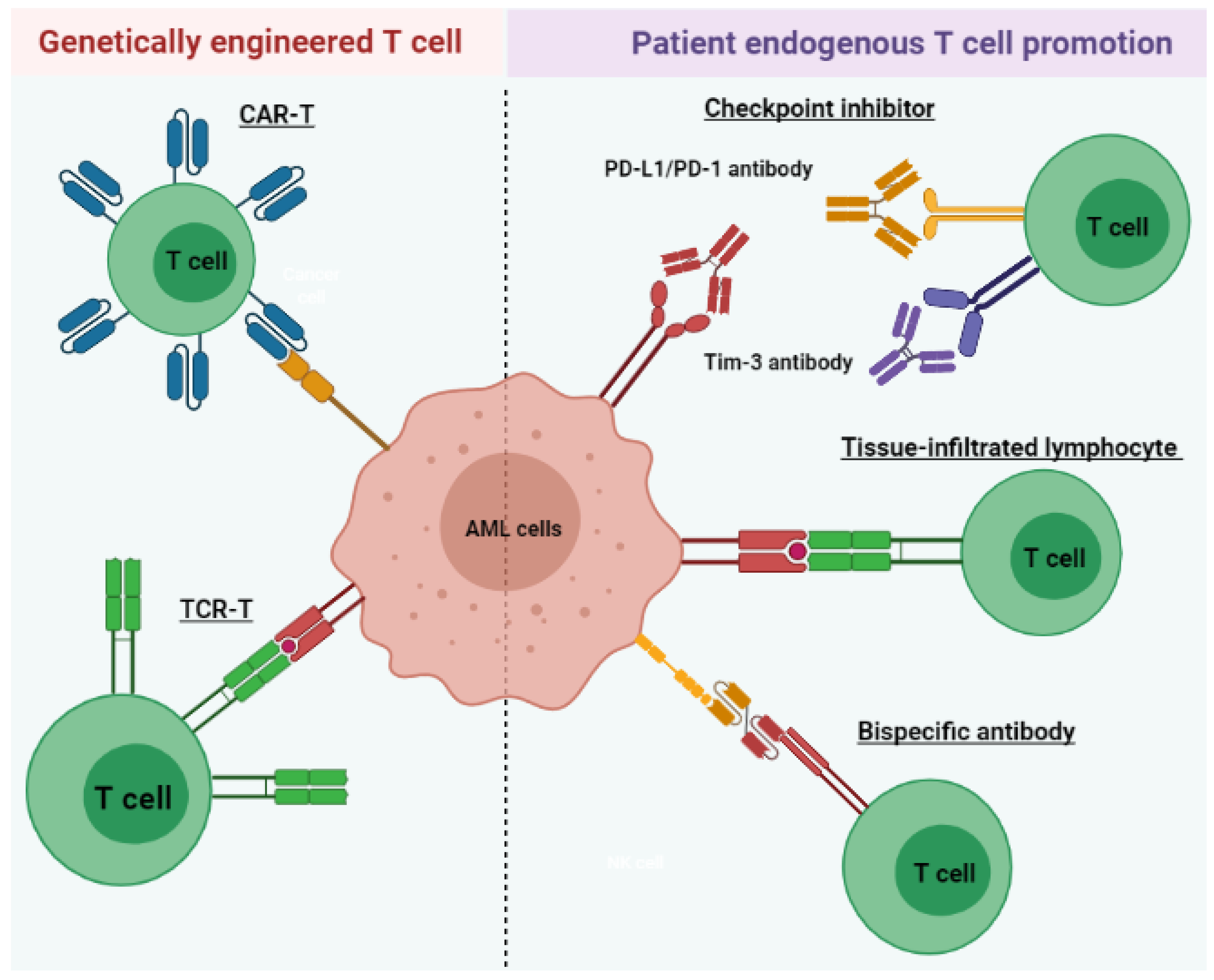
| Name | Target CheckPoint/Antigen | Clinical Trial Start Time | Trial Number/Phase | Disease | Status |
|---|---|---|---|---|---|
| T cell-recruiting bispecific antibody | CD3 and CD33 | 2015 | NCT02520427/Phase 1 | Relapsed/Refractory AML | Recruiting; estimated completion on 28 February 2023 |
| 2017 | NCT03224819/Phase 1 | AML | Active; estimated completion on 21 March 2022 | ||
| 2017 | NCT03144245/Phase 1 | AML | Completed on 5 November 2020; no result posted | ||
| 2019 | NCT03915379/Phase 1 | AML and myelodysplastic syndromes | Recruiting; estimated completion on 26 October 2022 | ||
| 2018 | NCT03516760//Phase 1 | Relapsed/Refractory AML | Active; estimated completion on 31 December 2020 | ||
| Immune checkpoint inhibitor | PD-1 | 2015 | NCT02397720/Phase 2 | AML | Recruiting; estimated completion on 30 April 2022 |
| 2016 | NCT02845297/Phase 2 | AML | Active; estimated completion in October 2021 | ||
| 2020 | NCT04214249/Phase 2 | AML | Not yet recruiting; estimated completion on 31 July 2024 | ||
| Chimeric antigen receptor T cell therapy | CD123 | 2015 | NCT02159495/Phase 1 | AML | Recruiting; estimated completion on 15 December 2021 |
| CD33/Lewis Y | 2013 | NCT01864902/Phase 1 and 2 | Relapsed/refractory AML | Unknown recruiting status; estimated completion in April 2017 | |
| Tumor-specific T cell receptor gene-transduced T cells | WT 1 | 2012 | NCT01640301/Phase 1 and 2 | Recurrent and secondary AML | Active; estimated completion on 1 September 2029 |
| 2002 | NCT00052520/Phase 1 and 2 | AML | Completed in June 2013; no result posted |
4. Conclusions
References
- Löwenberg, B.; Downing, J.R.; Burnett, A. Acute myeloid leukemia. N. Engl. J. Med. 1999, 341, 1051–1062.
- Cai, S.F.; Levine, R.L. Genetic and epigenetic determinants of AML pathogenesis. Semin. Hematol. 2019, 56, 84–89.
- Pelcovits, A.; Niroula, R. Acute Myeloid Leukemia: A Review. Rhode Isl. Med J. 2020, 103, 38–40.
- Kantarjian, H. Acute myeloid leukemia—Major progress over four decades and glimpses into the future. Am. J. Hematol. 2016, 91, 131–145.
- Swaminathan, M.; Wang, E.S. Novel therapies for AML: A round-up for clinicians. Expert Rev. Clin. Pharmacol. 2020, 13, 1389–1400.
- Medeiros, B.C.; Chan, S.M.; Daver, N.G.; Jonas, B.A.; Pollyea, D.A. Optimizing survival outcomes with post-remission therapy in acute myeloid leukemia. Am. J. Hematol. 2019, 94, 803–811.
- Mueller, B.U.; Seipel, K.; Bacher, U.; Pabst, T. Autologous Transplantation for Older Adults with AML. Cancers 2018, 10, 340.
- Takami, A. Hematopoietic stem cell transplantation for acute myeloid leukemia. Int. J. Hematol. 2018, 107, 513–518.
- Kassim, A.A.; Savani, B.N. Hematopoietic stem cell transplantation for acute myeloid leukemia: A review. Hematol. Oncol. Stem Cell Ther. 2017, 10, 245–251.
- Blazar, B.R.; Hill, G.R.; Murphy, W.J. Dissecting the biology of allogeneic HSCT to enhance the GvT effect whilst minimizing GvHD. Nat. Rev. Clin. Oncol. 2020, 17, 475–492.
- Sweeney, C.; Vyas, P. The Graft-Versus-Leukemia Effect in AML. Front. Oncol. 2019, 9, 1217.
- Radujkovic, A.; Guglielmi, C.; Bergantini, S.; Iacobelli, S.; van Biezen, A.; Milojkovic, D.; Gratwohl, A.; Schattenberg, A.V.; Verdonck, L.F.; Niederwieser, D.W.; et al. Donor Lymphocyte Infusions for Chronic Myeloid Leukemia Relapsing after Allogeneic Stem Cell Transplantation: May We Predict Graft-versus-Leukemia Without Graft-versus-Host Disease? Biol. Blood Marrow Transplant. 2015, 21, 1230–1236.
- Orti, G.; Barba, P.; Fox, L.; Salamero, O.; Bosch, F.; Valcarcel, D. Donor lymphocyte infusions in AML and MDS: Enhancing the graft-versus-leukemia effect. Exp. Hematol. 2017, 48, 1–11.
- Ramachandran, V.; Kolli, S.S.; Strowd, L.C. Review of Graft-Versus-Host Disease. Dermatol. Clin. 2019, 37, 569–582.
- Ferrara, J.L.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561.
- Orleans-Lindsay, J.K.; Barber, L.D.; Prentice, H.G.; Lowdell, M.W. Acute myeloid leukaemia cells secrete a soluble factor that inhibits T and NK cell proliferation but not cytolytic function--implications for the adoptive immunotherapy of leukaemia. Clin. Exp. Immunol. 2001, 126, 403–411.
- Pyzer, A.R.; Stroopinsky, D.; Rajabi, H.; Washington, A.; Tagde, A.; Coll, M.; Fung, J.; Bryant, M.P.; Cole, L.; Palmer, K.; et al. MUC1-mediated induction of myeloid-derived suppressor cells in patients with acute myeloid leukemia. Blood 2017, 129, 1791–1801.
- Deng, M.; Gui, X.; Kim, J.; Xie, L.; Chen, W.; Li, Z.; He, L.; Chen, Y.; Chen, H.; Luo, W.; et al. LILRB4 signalling in leukaemia cells mediates T cell suppression and tumour infiltration. Nature 2018, 562, 605–609.
- Coles, S.J.; Hills, R.K.; Wang, E.C.Y.; Burnett, A.K.; Man, S.; Darley, R.L.; Tonks, A. Increased CD200 expression in acute myeloid leukemia is linked with an increased frequency of FoxP3+ regulatory T cells. Leukemia 2012, 26, 2146–2148.
- Ustun, C.; Miller, J.S.; Munn, D.H.; Weisdorf, D.J.; Blazar, B.R. Regulatory T cells in acute myelogenous leukemia: Is it time for immunomodulation? Blood 2011, 118, 5084–5095.
- Zhou, Q.; Bucher, C.; Munger, M.E.; Highfill, S.L.; Tolar, J.; Munn, D.H.; Levine, B.L.; Riddle, M.; June, C.H.; Vallera, D.A.; et al. Depletion of endogenous tumor-associated regulatory T cells improves the efficacy of adoptive cytotoxic T-cell immunotherapy in murine acute myeloid leukemia. Blood 2009, 114, 3793–3802.
- Shenghui, Z.; Yixiang, H.; Jianbo, W.; Kang, Y.; Laixi, B.; Yan, Z.; Xi, X. Elevated frequencies of CD4⁺ CD25⁺ CD127lo regulatory T cells is associated to poor prognosis in patients with acute myeloid leukemia. Int. J. Cancer 2011, 129, 1373–1381.
- Tian, T.; Yu, S.; Liu, L.; Xue, F.; Yuan, C.; Wang, M.; Ji, C.; Ma, D. The Profile of T Helper Subsets in Bone Marrow Microenvironment Is Distinct for Different Stages of Acute Myeloid Leukemia Patients and Chemotherapy Partly Ameliorates These Variations. PLoS ONE 2015, 10, e0131761.
- Knaus, H.A.; Berglund, S.; Hackl, H.; Blackford, A.L.; Zeidner, J.F.; Montiel-Esparza, R.; Mukhopadhyay, R.; Vanura, K.; Blazar, B.R.; Karp, J.E.; et al. Signatures of CD8+ T cell dysfunction in AML patients and their reversibility with response to chemotherapy. JCI Insight 2018, 3, e120974.
- Tan, J.; Chen, S.; Lu, Y.; Yao, D.; Xu, L.; Zhang, Y.; Yang, L.; Chen, J.; Lai, J.; Yu, Z.; et al. Higher PD-1 expression concurrent with exhausted CD8+ T cells in patients with de novo acute myeloid leukemia. Chin. J. Cancer Res. 2017, 29, 463–470.





