Acute myeloid leukemia (AML) is a heterogeneous disease associated with various alterations in T cell phenotype and function leading to an abnormal cell population, ultimately leading to immune exhaustion. However, restoration of T cell function allows for the execution of cytotoxic mechanisms against leukemic cells in AML patients. Therefore, long-term disease control, which requires multiple therapeutic approaches, includes those aimed at the re-establishment of cytotoxic T cell activity. AML treatments that harness the power of T lymphocytes against tumor cells have rapidly evolved over the last 3 to 5 years through various stages of preclinical and clinical development. These include tissue-infiltrated lymphocytes (TILs), bispecific antibodies, immune checkpoint inhibitors (ICIs), chimeric antigen receptor T (CAR-T) cell therapy, and tumor-specific T cell receptor gene-transduced T (TCR-T) cells.
- acute myeloid leukemia
- T cell immunotherapy
- T cell alteration
- CAR-T
- TCR-T
1. Introduction
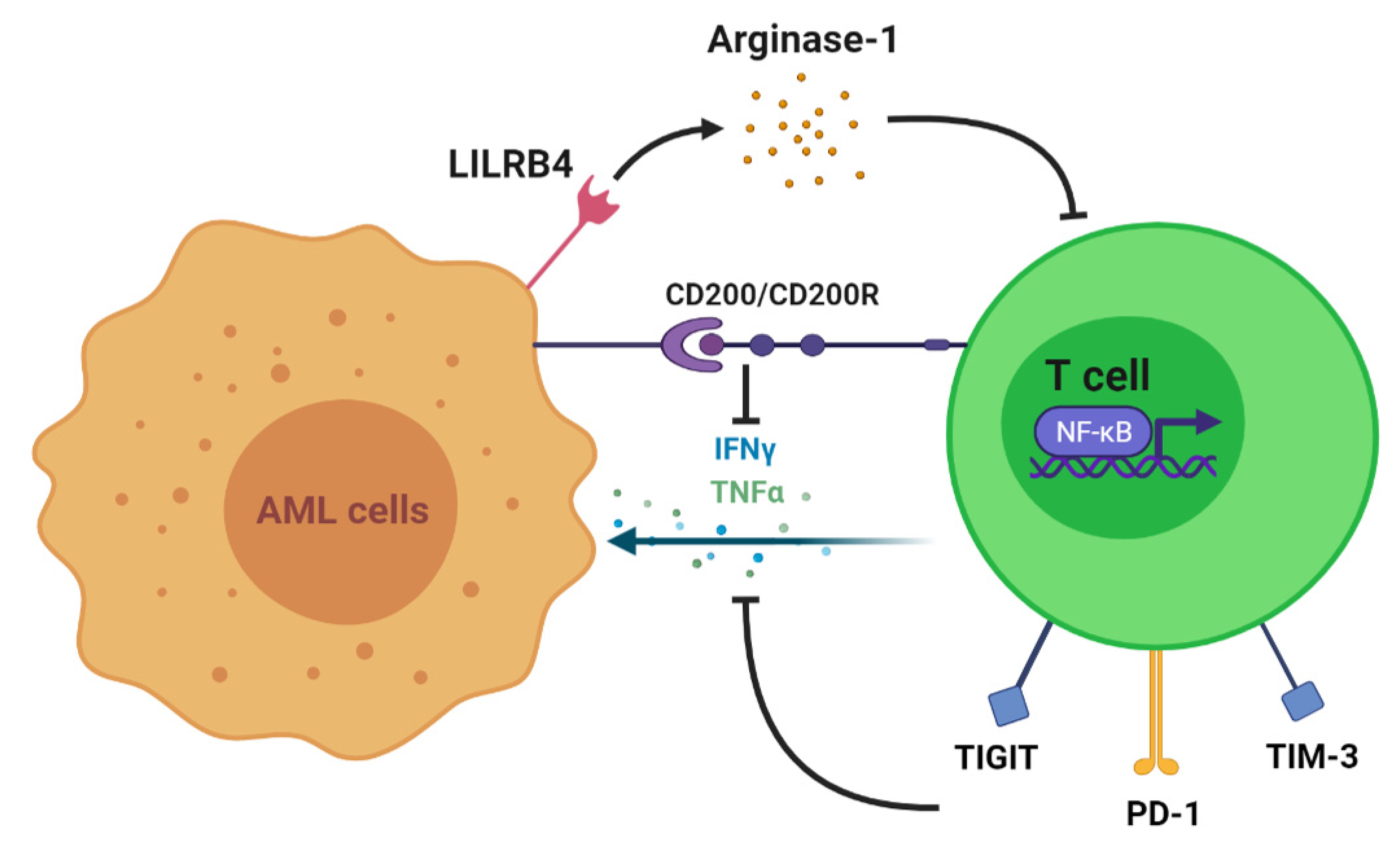
2. T Cell Alteration in AML
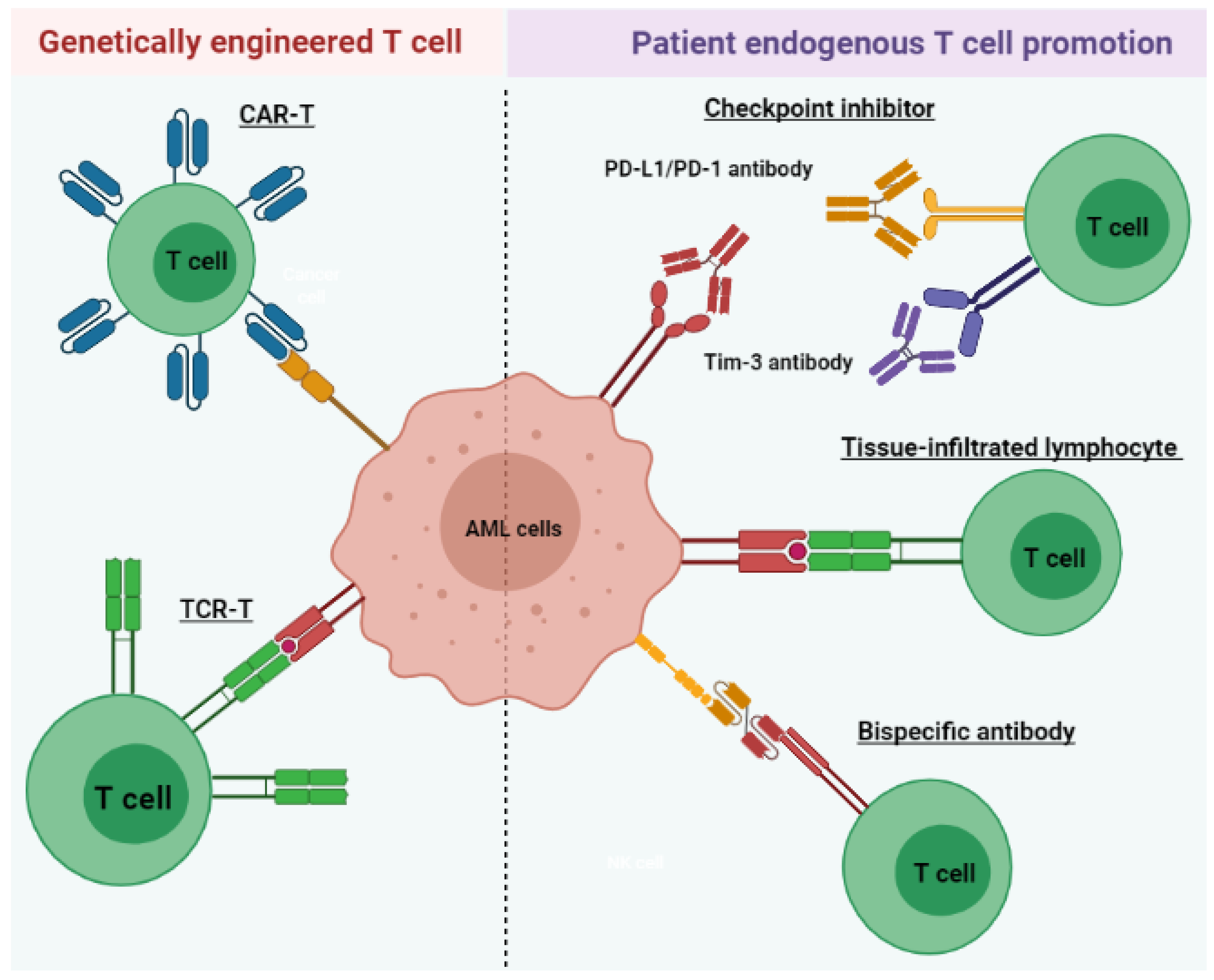
3. T Cell Immunotherapy
| Name | Target CheckPoint/Antigen | Clinical Trial Start Time | Trial Number/Phase | Disease | Status |
|---|---|---|---|---|---|
| T cell-recruiting bispecific antibody | CD3 and CD33 | 2015 | NCT02520427/Phase 1 | Relapsed/Refractory AML | Recruiting; estimated completion on 28 February 2023 |
| 2017 | NCT03224819/Phase 1 | AML | Active; estimated completion on 21 March 2022 | ||
| 2017 | NCT03144245/Phase 1 | AML | Completed on 5 November 2020; no result posted | ||
| 2019 | NCT03915379/Phase 1 | AML and myelodysplastic syndromes | Recruiting; estimated completion on 26 October 2022 | ||
| 2018 | NCT03516760//Phase 1 | Relapsed/Refractory AML | Active; estimated completion on 31 December 2020 | ||
| Immune checkpoint inhibitor | PD-1 | 2015 | NCT02397720/Phase 2 | AML | Recruiting; estimated completion on 30 April 2022 |
| 2016 | NCT02845297/Phase 2 | AML | Active; estimated completion in October 2021 | ||
| 2020 | NCT04214249/Phase 2 | AML | Not yet recruiting; estimated completion on 31 July 2024 | ||
| Chimeric antigen receptor T cell therapy | CD123 | 2015 | NCT02159495/Phase 1 | AML | Recruiting; estimated completion on 15 December 2021 |
| CD33/Lewis Y | 2013 | NCT01864902/Phase 1 and 2 | Relapsed/refractory AML | Unknown recruiting status; estimated completion in April 2017 | |
| Tumor-specific T cell receptor gene-transduced T cells | WT 1 | 2012 | NCT01640301/Phase 1 and 2 | Recurrent and secondary AML | Active; estimated completion on 1 September 2029 |
| 2002 | NCT00052520/Phase 1 and 2 | AML | Completed in June 2013; no result posted |
4. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/cells10123376
