
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Khalid Rehman Hakeem | + 3198 word(s) | 3198 | 2021-12-15 05:20:19 | | | |
| 2 | Conner Chen | Meta information modification | 3198 | 2021-12-24 04:07:28 | | |
Video Upload Options
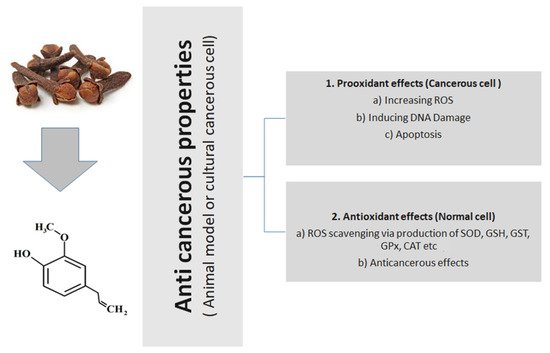
Clove (Syzygium aromaticum L.) (Family Myrtaceae) is a highly prized spice that has been historically utilized as a food preservative and for diverse medical uses. It is reckoned amongst the valued sources of phenolics. Among diverse active components, eugenol, the principal active component of S. aromaticum, has optimistic properties comprising antioxidant, anti-inflammatory, and anticancer actions. Eugenol (4-allyl-2-methoxyphenol) is a musky oil that is mainly obtained from clove. It has long been utilized all over the world as a result of its broad properties like antioxidant, anticancer, anti-inflammatory, and antimicrobial activities. Anticancer effects of eugenol are accomplished by various mechanisms like inducing cell death, cell cycle arrest, inhibition of migration, metastasis, and angiogenesis on several cancer cell lines. Besides, eugenol might be utilized as an adjunct remedy for patients who are treated with conventional chemotherapy. This combination leads to a boosted effectiveness with decreased toxicity.
1. Lung Cancer
2. Colon Cancer
3. Gastric Cancer
4. Cervical Cancer
5. Melanoma
6. Breast Cancer
References
- Fangjun, L.; Zhijia, Y. Tumor suppressive roles of eugenol in human lung cancer cells. Thorac. Cancer 2017, 9, 25–29.
- Fathy, M.; Fawzy, M.; Hintzsche, H.; Nikaido, T.; Dandekar, T.; Othman, E.M. Eugenol Exerts Apoptotic Effect and Modulates the Sensitivity of HeLa Cells to Cisplatin and Radiation. Molecules 2019, 24, 3979.
- Choudhury, P.; Barua, A.; Roy, A.; Pattanayak, R.; Bhattacharyya, M.; Saha, P. Eugenol emerges as an elixir by targeting β-catenin, the central cancer stem cell regulator in lung carcinogenesis: An in vivo and in vitro rationale. Food Funct. 2020, 12, 1063–1078.
- De Cássia, R.; da Silveira, S.A.; Rade, L.N.; de Sousa, D.P. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon, (Cinnamomum osmophloeum) twigs. Bioresour. Technol. 2008, 99, 3908–3913.
- Petrocelli, G.; Farabegoli, F.; Valerii, M.C.; Giovannini, C.; Sardo, A.; Spisni, E. Molecules Present in Plant Essential Oils for Prevention and Treatment of Colorectal Cancer (CRC). Molecules 2021, 26, 885.
- Jaganathan, S.K.; Supriyanto, E. Antiproliferative and Molecular Mechanism of Eugenol-Induced Apoptosis in Cancer Cells. Molecules 2012, 17, 6290–6304.
- Jaganathan, S.K.; Mazumdar, A.; Mondhe, D.; Mandal, M. Apoptotic effect of eugenol in human colon cancer cell lines. Cell Biol. Int. 2011, 35, 607–615.
- Towaha, J. Manfaat eugenol cengkeh dalam berbagai industri di Indonesia. Perspektif 2012, 11, 79–90.
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947.
- Manikandan, P.; Murugan, R.S.; Priyadarsini, R.V.; Vinothini, G.; Nagini, S. Eugenol induces apoptosis and inhibits invasion and angiogenesis in a rat model of gastric carcinogenesis induced by MNNG. Life Sci. 2010, 86, 936–941.
- Manikandan, P.; Vinothini, G.; Priyadarsini, R.V.; Prathiba, D.; Nagini, S. Eugenol inhibits cell proliferation via NF-κB suppression in a rat model of gastric carcinogenesis induced by MNNG. Investig. New Drugs 2011, 29, 110–117.
- Small, W., Jr.; Bacon, M.A.; Bajaj, A.; Chuang, L.T.; Fisher, B.J.; Harkenrider, M.M.; Jhingran, A.; Kitchener, H.C.; Mileshkin, L.R.; Viswanathan, A.N.; et al. Cervical cancer: A global health crisis. Cancer 2017, 123, 2404–2412.
- Jiang, Z.; Song, Q.; Zeng, R.; Li, J.; Li, J.; Lin, X.; Chen, X.; Zhang, J.; Zheng, Y. MicroRNA-218 inhibits EMT, migration and invasion by targeting SFMBT1 and DCUN1D1 in cervical cancer. Onco Targets Ther. 2016, 7, 45622.
- Lee, M.Y.; Shen, M.R. Epithelial-mesenchymal transition in cervical carcinoma. Am. J. Transl. Res. 2012, 4, 1.
- Myong, N.H. Loss of E-cadherin and acquisition of vimentin in epithelial-mesenchymal transition are noble indicators of uterine cervix cancer progression. Korean J. Pathol. 2012, 46, 341.
- Xu, W.; Yang, Z.; Lu, N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh Mig 2015, 9, 317–324.
- Ha, G.H.; Park, J.S.; Breuer, E.K. TACC3 promotes epithelial–mesenchymal transition (EMT) through the activation of PI3K/Akt and ERK signaling pathways. Cancer Lett. 2013, 332, 63–73.
- Bermudez, A.; Bhatla, N.; Leung, E. Cancer of the cervix uteri. Int. J. Gynecol Obstet 2015, 131, S88–S95.
- Van Minh, H.; My, N.T.; Jit, M. Cervical cancer treatment costs and cost-effectiveness analysis of human papillomavirus vaccination in Vietnam: A PRIME modeling study. BMC Health Serv. Res. 2017, 17, 353.
- Permatasari, H.K.; Kusuma, I.D.; Mayangsari, E. Minyak Cengkeh (Syzygium aromaticum) menginduksi apoptosis pada sel kanker servik HeLa melalui peningkatan kadar protein p53. J. Kedokt Brawijaya 2019, 30, 185–190.
- Permatasari, H.K.; Effendi, A.B.; Qhabibi, F.R.; Fawwaz, F.; Dominique, A. Eugenol isolated from Syzygium aromaticum inhibits HeLa cancer cell migration by altering epithelial-mesenchymal transition protein regulators. J. Appl. Pharm. Sci. 2021, 11, 49–53.
- Petrova, Y.I.; Schecterson, L.; Gumbiner, B.M. Roles for E-cadherin cell surface regulation in cancer. Mol. Biol. Cell 2016, 27, 3233–3244.
- Yi, J.-L.; Shi, S.; Shen, Y.-L.; Wang, L.; Chen, H.-Y.; Zhu, J.; Ding, Y. Myricetin and methyl eugenol combination enhances the anticancer activity, cell cycle arrest and apoptosis induction of cis-platin against HeLa cervical cancer cell lines. Int. J. Clin. Exp. Pathol. 2015, 8, 1116–1127.
- Kachhap, S.; Pratap, A.; Toppo, D.S. Abdominal malignant melanoma: Rare case report. Int. J. Scientific. Res. 2019, 8.
- Miller, A.J.; Mihm, M.C., Jr. Melanoma. N. Engl. J. Med. 2006, 355, 51–65.
- Pisano, M.; Pagnan, G.; Loi, M.; Mura, M.E.; Tilocca, M.G.; Palmieri, G.; Fabbri, D.; Dettori, M.A.; Delogu, G.; Ponzoni, M.; et al. Antiproliferative and pro-apoptotic activity of eugenol-related biphenyls on malignant melanoma cells. Mol Cancer 2007, 6, 8–20.
- Miyazawa, M.; Hisama, M. Suppression of chemical mutagen-induced SOS response by alkylphenols from clove (Syzygium aromaticum) in the Salmonella typhimurium TA1535/pSK1002 umu test. J. Agric. Food Chem. 2001, 49, 4019–4025.
- Bendre, R.S.; Rajput, J. Outlooks on medicinal properties of eugenol and its synthetic derivatives. Nat. Prod. Chem. Resh. 2016, 4, 2.
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M. Eugenol—from the remote Maluku Islands to the Int market place: A review of a remarkable and versatile molecule. Molecules 2012, 17, 6953–6981.
- Kim, G.C.; Choi, D.S.; Lim, J.S. Caspases-dependent apoptosis in human melanoma cell by eugenol. Korean J. Anat. 2006, 39, 245–253.
- Ghosh, R.; Nadiminty, N.; Fitzpatrick, J.E.; Alworth, W.L.; Slaga, T.J.; Kumar, A.P. Eugenol causes melanoma growth suppression through inhibition of E2F1 transcriptional activity. J. Biol. Chem. 2005, 280, 5812–5819.
- Shin, S.H.; Park, J.H.; Kim, G.C. The mechanism of apoptosis induced by eugenol in human osteosarcoma cells. J. Korean Oral Maxillofac Surg. 2007, 33, 20–27.
- Cassano, R.; Cuconato, M.; Calviello, G.; Serini, S.; Trombino, S. Recent advances in nanotechnology for the treatment of melanoma. Molecules 2021, 26, 785.
- Hafeez, A.; Kazmi, I. Dacarbazine nanoparticle topical delivery system for the treatment of melanoma. Sci. Rep. 2017, 7, 1–10.
- Pinho, J.O.; Matias, M.; Gaspar, M.M. Emergent Nanotechnological Strategies for Systemic Chemotherapy against Melanoma. Nanomaterial 2019, 9, 1455.
- Mishra, H.; Mishra, P.K.; Iqbal, Z.; Jaggi, M.; Madaan, A.; Bhuyan, K.; Gupta, N.; Gupta, N.; Vats, K.; Verma, R. Co-Delivery of Eugenol and Dacarbazine by Hyaluronic Acid-Coated Liposomes for Targeted Inhibition of Survivin in Treatment of Resistant Metastatic Melanoma. Pharmaceutics 2019, 11, 163.
- Dwivedi, V.; Shrivastava, R.; Hussain, S.; Ganguly, C.; Bharadwaj, M. Comparative anticancer potential of clove (Syzygium aromaticum)—an Indian spice—against cancer cell lines of various anatomical origin. Asian Pac. J. Cancer Prev. 2011, 12, 1989–1993.
- 1Kumar, P.S.; Febriyanti, R.M.; Sofyan, F.F.; Luftimas, D.E.; Abdulah, R. Anticancer potential of Syzygium aromaticum L. in MCF-7 human breast cancer cell lines. Pharmacog. Res. 2014, 6, 350.
- Nejad, S.M.; Özgüneş, H.; Başaran, N. Pharmacological and Toxicological Properties of Eugenol. Turk. J. Pharm. Sci. 2017, 14, 201–206.
- Youlden, D.R.; Cramb, S.M.; Yip, C.H.; Baade, P.D. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Bio. Med. 2014, 11, 101–115.
- Al-Sharif, I.; Remmal, A.; Aboussekhra, A. Eugenol triggers apoptosis in breast cancer cells through E2F1/survivin down-regulation. BMC Cancer 2013, 13, 1–10.
- Abdullah, M.L.; Hafez, M.M.; Al-Hoshani, A.; Al-Shabanah, O. Anti-metastatic and anti-proliferative activity of eugenol against triple negative and HER2 positive breast cancer cells. BMC Com. Alt Med. 2018, 18, 1–11.
- Vidhya, N.; Devaraj, S.N. Induction of apoptosis by eugenol in human breast cancer cells. Indian J. Exp. Bio. 2011, 49, 871–878.
- Anuj, G.; Sanjay, S. Eugenol: A potential phytochemical with multifaceted therapeutic activities. Pharmacology 2010, 2, 108–120.
- Abdullah, M.L.; Al-Shabanah, O.; Hassan, Z.K.; Hafez, M.M. Eugenol-Induced Autophagy and Apoptosis in Breast Cancer Cells via PI3K/AKT/FOXO3a Pathway Inhibition. Int. J. Mol. Sci. 2021, 22, 9243.




