Breast cancer is a serious life-threatening health problem for millions of women around the world every year. It is classified as the second most common cancer in women, and is ranked fourth for cancer-related deaths globally
[39][37]. In women, mammary epithelial cells are controlled by keeping a balance between the cell cycle and apoptosis. Disruption of this causes an increase in mammary epithelial cells, lastly leading to breast cancer
[40][107]. The majority of chemotherapy agents that are presently utilized as a means of treatment of this malady are extremely lethal and have long-term side effects. Thus, new anticancer medicines with greater efficacy and specificity are immediately required. The influence of eugenol on apoptotic and procarcinogenic proteins, both in vitro and in tumor xenografts, was evaluated via immunoblotting and RT-PCR was utilized to determine the influence of eugenol on E2F1 and survivin mRNA levels. Al-Sharif et al. examined the influence of eugenol on cell proliferation by means of a real-time electronic cell detection system. Low-dose eugenol (2 µM) has explicit toxicity against several breast cancer cells. This lethal influence was chiefly facilitated by induction of the internal apoptotic pathway and potent downregulation of E2F1 and its downstream antiapoptosis target, surviving, regardless of p53 and ERa status. Eugenol also inhibited many other breast cancer-related oncogenes, like NF-κB and cyclin D1. Furthermore, eugenol upregulated the multipurpose cyclin-dependent kinase inhibitor p21
WAF1 protein and inhibited the proliferation of breast cancer cells in a p53-independent manner. Significantly, these antiproliferative and pro-apoptotic influences have also been noticed in vivo in xenografted human breast cancers. Therefore, eugenol displays anticancer characteristics both in vitro and in vivo, demonstrating that it might be utilized to strengthen adjuvant breast cancer treatment by targeting the E2F1/surviving pathway, particularly for the less-sensitive triple-negative subtype of the disorder
[41][87]. Abdullah et al. investigated the probability of using eugenol as an antimetastatic and antiproliferative compound against MDA-MB-231 and SK-BR-3 breast cancer cells. Treatment with 4 and 8 μM eugenol for 48 h substantially hindered cell proliferation of MDA-MB-231, with an inhibition rate of 76.4%, while 5 and 10 μM of eugenol for 48 h substantially hindered the proliferation of SK-BR-3 cells with an inhibition rate of 68.1%. Eugenol-treated cells demonstrated substantially reduced expression of MMP2 and MMP9 and an insignificant rise in the expression of TIMP1 in HER2-positive and triple-negative breast cancer cells. Eugenol greatly raised the proportion of MDA-MB-231 and SK-BR-3 cells in late apoptosis and elevated Caspase3, Caspase7, and Caspase9 expression
[42][88]. Vidhya and Devaraj
[43][108] verified that breast cancer cells (MCF-7) experience the potent antimutagenic action of eugenol. Eugenol is both time and dose dependent when defeating the proliferation of MCF-7 cells
[43][44][108,109]. Furthermore, Pisano et al.
[26][80] described the antiproliferative activity of eugenol-related biphenyl (S)-6,60-dibromo-dehydrodieugenol, via induction of apoptosis.
The modification of autophagy may enhance either the survival or apoptosis of cancer cells. Abdullah et al. treated triple-negative (MDA-MB-231) and HER2-positive (SK-BR-3) breast cancer cell lines with varied eugenol doses. They examined apoptosis via a flow-cytometry technique, whereas autophagy via acridine orange. They investigated the influence of eugenol on the gene and protein expression levels of autophagy and apoptotic genes. Treating cells with varying doses of eugenol substantially hindered cell proliferation. The protein levels of AKT serine/threonine kinase 1 (AKT), forkhead box O3 (FOXO3a), cyclin-dependent kinase inhibitor 1A (p21), cyclin-dependent kinase inhibitor (p27), and Caspase-3 and -9 increased considerably in eugenol-treated cells. Furthermore, eugenol triggered autophagy through upregulation of microtubule-associated protein 1 light chain 3 (LC3) expression levels and downregulation of nucleoporin 62 (NU p62) expression. Eugenol is a potential natural anticancer agent against triple-negative and HER2-positive breast cancer. It seems to act via targeting of the caspase pathway and via triggering of autophagic cell death
[45][28].
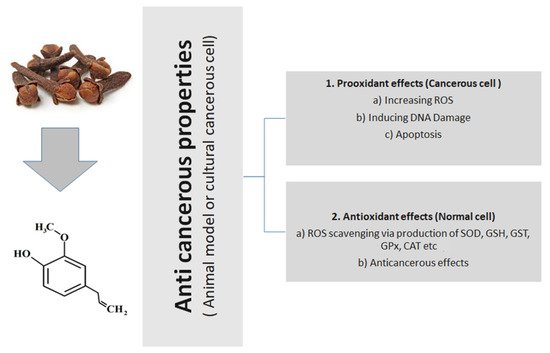
Figure 1 presents the general action of eugenol, tried and tested in animal or cultural cancerous cell models.
Figure 1.
General anticancerous action of eugenol (tried and tested in animal or cultural cancerous cell models).