Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Takashi Nakayama | + 2553 word(s) | 2553 | 2021-12-15 03:31:35 | | | |
| 2 | Yvaine Wei | Meta information modification | 2553 | 2021-12-23 09:38:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nakayama, T. Roles of Chemokines/Chemokine Receptors in Tumor Immunity. Encyclopedia. Available online: https://encyclopedia.pub/entry/17492 (accessed on 07 February 2026).
Nakayama T. Roles of Chemokines/Chemokine Receptors in Tumor Immunity. Encyclopedia. Available at: https://encyclopedia.pub/entry/17492. Accessed February 07, 2026.
Nakayama, Takashi. "Roles of Chemokines/Chemokine Receptors in Tumor Immunity" Encyclopedia, https://encyclopedia.pub/entry/17492 (accessed February 07, 2026).
Nakayama, T. (2021, December 23). Roles of Chemokines/Chemokine Receptors in Tumor Immunity. In Encyclopedia. https://encyclopedia.pub/entry/17492
Nakayama, Takashi. "Roles of Chemokines/Chemokine Receptors in Tumor Immunity." Encyclopedia. Web. 23 December, 2021.
Copy Citation
Various immune cells are involved in host immune responses to cancer. T-helper (Th) 1 cells, cytotoxic CD8+ T cells, and natural killer cells are the major effector cells in anti-tumor immunity, whereas cells such as regulatory T cells and myeloid-derived suppressor cells are negatively involved in anti-tumor immunity. Th2 cells and Th17 cells have been shown to have both pro-tumor and anti-tumor activities. The migratory properties of various immune cells are essential for their function and critically regulated by the chemokine superfamily.
chemokine
chemokine receptor
Th1
Th2
Th17
regulatory T
macrophage
neutrophil
tumor microenvironment
1. Introduction
Tumor immunity is initiated by the recognition of tumor antigens by the immune system [1]. Antigen priming and effector cell differentiation are regulated by complex processes involving various cell populations, cytokines, and chemokines [1][2][3]. While CD4+ helper T cells are known to orchestrate immune responses, CD4+ T cells are heterogenous and composed of various functional subsets including T-helper (Th) 1 cells, Th2 cells, Th17 cells, and regulatory T (Treg) cells [4][5]. These T cell subsets are characterized by the secretion of signature cytokines and differentially involved in tumor immunity [4][5]. Furthermore, CD8+ cytotoxic lymphocytes (CTLs) and natural killer (NK) cells play direct roles in the elimination of tumor cells. Tumor tissues are also highly enriched with innate immune cells such as myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), and tumor-associated neutrophils (TANs) [6][7]. These various immune cells are known to express characteristic chemokine receptors and are elaborately regulated in their migration and tissue localization by respective chemokine ligands [8][9][10]. Accordingly, some chemokines and their receptors may be involved in the elimination of tumor cells by recruiting anti-tumor effector cells, whereas others may promote tumor progression by attracting immunosuppressive cells. In addition, some chemokines and their receptors are known to play critical roles in the interactions of dendric cells (DCs) and T cells [11][12][13][14]. Thus, the chemokine superfamily has a multifaceted role in host tumor immunity.
2. Induction of Tumor-Specific T Cell Responses
T cell-mediated anti-tumor immunity is thought to be achieved by a multistep process called the cancer-immunity cycle [1]. It includes the following seven steps: (1) release of cancer antigens, (2) cancer antigen presentation, (3) priming and activation, (4) trafficking of T cells to tumors, (5) infiltration of T cells into tumors, (6) recognition of cancer cells by T cells, and (7) killing of cancer cells. Thus, the cycle is initiated by the uptake of tumor antigens, including tumor-associated antigens and neoantigens, by DCs, the professional antigen-presenting cells. Tumor antigen-captured DCs then migrate into the draining lymph nodes where recirculating naïve T cells and memory T cells scan antigenic peptides presented by DCs in association with class I and class II major histocompatibility complex (MHC) molecules (Figure 1). The CCL19/CCL21-CCR7 axis is known to play a pivotal role in the migratory activities of DCs and recirculating T cells [1][15]. While immature DCs in peripheral tissues dominantly express CCR6, the surface expression of CCR7 is upregulated upon antigen-loading and DC maturation [1][15]. Since CCL21 is abundantly produced by lymphatic vessels, CCR7-expressing DCs initiate trans-lymphatic migration and home into T cell areas of the draining lymph nodes where matured DCs start producing CCL19 (Figure 1) [3][16][17]. The lymph nodes also have unique vascular structures called high endothelial venules (HEVs), which produce CCL21 and function as the gateways for recirculating naïve T cells and memory T cells that commonly express CCR7 (Figure 1) [18][19]. In addition, although CCL19 is not produced by HEVs, it is displayed on the luminal surfaces of HEVs by transcytosis [20]. After homing into the lymph nodes, naïve T cells and memory T cells further migrate toward CCL19-producing mature DCs localized in the T cell areas [1][15]. Upon encounter with cognate antigenic peptides presented by mature DCs, antigen-specific naïve T cells proliferate and differentiate into various effector T cell subsets in accordance with the local cytokine milieu, whereas memory T cells start rapid expansions for recall immune responses [21]. Furthermore, conventional DCs have two subtypes known as type 1 (cDC1s) and type 2 (cDC2s) [22]. It is now known that cDC1s preferentially induce the differentiation of naïve CD4+ T cells and CD8+ T cells into Th1 cells and CTLs, respectively [3][16][17], whereas cDC2s preferentially induce the differentiation of naïve CD4+ T cells into Th2 cells and Th17 cells (Figure 1) [3][16][17]. Importantly, cDC1s selectively express XCR1 and are the most efficient DCs in the cross-presentation of exogenous antigens to CD8+ T cells [22]. Of note, while cDC1s activate CCR7-expressing naïve CD8+ T cells [22], activated CD8+ T cells in turn produce XCL1, the ligand of XCR1 (Figure 1) [22]. This further promotes the interactions of CD8+ T cells and cDC1s, leading to full differentiation of effector CTLs [22]. In secondary immune responses, mature DCs also produce CCL3, CCL4, CCL5, CCL17, CCL22, CXCL9, CXCL10, and CXCL11 [22]. Since Th1 cells express CCR5 and CXCR3, while Th2 cells, Th17 cells, and Treg cells express CCR4 [22], these chemokine–chemokine receptor axes contribute to the rapid expansion of effector T cells (Figure 1).
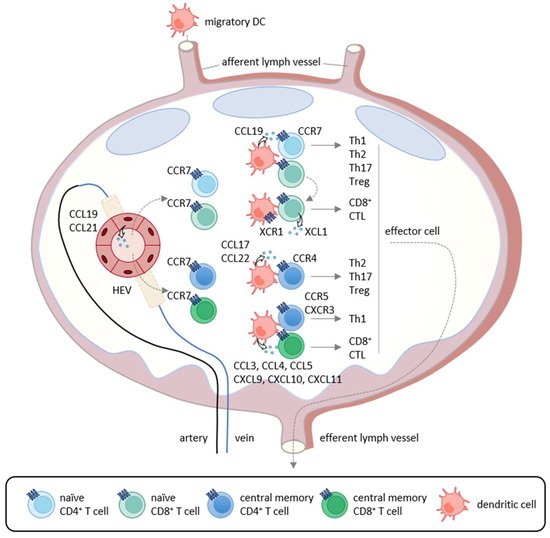
Figure 1. Chemokine-mediated T cell immune responses in the lymph node. In primary immune responses, antigen-captured DCs migrate into the draining lymph nodes via afferent lymphatics using the CCL19/CCL21-CCR7 axis. In the lymph nodes, mature DCs produce CCL19 and interact with recirculating CCR7-expressing naïve CD4+ T cells that home into the lymph nodes via the high endothelial venules (HEVs). If stimulated by cognate antigenic peptides presented by mature DCs, naïve T cells differentiate into Th1 cells, Th2 cells, Th17 cells, and Treg cells according to the local cytokine milieu.
3. Th1 Cell, CTL, and NK Cell
Th1 cells are involved in cellular immunity by secreting Th1-type cytokines such as interferon (IFN)-γ, Interleukin (IL)-2 and tumor necrosis factor (TNF)-α [23][24]. Th1-type cytokines promote the differentiation of naïve CD8+ T cells into CTLs [23][24] and also enhance the cytotoxic activity of CD8+ T cells and NK cells [25][26]. CD8+ T cells and NK cells are able to directly eliminate tumor cells by secreting cytotoxic molecules such as perforin and granzyme. NK cells are also able to induce apoptosis in tumor cells via TNF family molecules such as FAS ligand (FASL) and TNF-related apoptosis-inducing ligand (TRAIL) [27]. Thus, Th1 cells, CD8+ T cells, and NK cells are the major effector cells in anti-tumor immunity. Indeed, infiltration of CD8+ T cells and NK cells correlates with better clinical outcomes and therapeutic responses in various types of cancer [28][29]. In addition, recent studies have revealed the existence of CD8+ T cell subsets such as IFN-γ-expressing Tc1 cells, IL-4-expressing Tc2 cells, IL-9-expressing Tc9 cells, IL-17-expressing Tc17 cells, and IL-22-expressing Tc22 cells [30][31][32][33][34]. Tc1, Tc2, and Tc22 cells produce high levels of perforin and granzyme and have high cytotoxic activity, while Tc9 and Tc17 produce low levels of cytotoxic molecules and have poor cytotoxic activity [35][36]. The respective roles of these various Tc subsets in tumor immunity remain to be seen.
4. Th2 Cell
Th2 cells are involved in humoral immunity by secreting Th2-type cytokines, such as IL-4, IL-5, IL-10, and IL-13 [37][38]. Th2 cells are shown to express CCR4, CCR8, and, to a lesser extent, CCR3 [39][40][41]. It has been reported that the expression levels of respective chemokine ligands are upregulated in some types of cancer, and Th2 cells are increased in tumor tissues and the draining lymph nodes [42]. Th2 cytokines inhibit the differentiation and function of Th1 cells, while Th1 cytokines inhibit the differentiation and function of Th2 cells [43]. Thus, Th2 cells may indirectly suppress anti-tumor immunity by negatively regulating Th1 responses. In particular, IL-10 is known to suppress Th1-mediated immune responses [44]. IL-4 has also been reported to enhance tumor growth by inhibiting apoptosis in a murine fibrosarcoma model [45]. On the other hand, it has also been demonstrated that adoptive transfer of tumor-specific Th2 cells protected mice against lethal challenge of murine myeloma and lymphoma through the induction of Th2-type inflammation in tumor tissues. However, Th2-mediated anti-tumor effects did not require B cells, NKT cells or CD8+ T cells and the mechanism was unknown [46]. Of note, Th2 cells are also known to secrete IL-31 [47]. Recently, it has been reported that IL-31-overexpressing murine breast carcinoma shows reduced tumor growth by inhibiting the activity of immunosuppressive cells such as Treg cells, MDSCs, and M2-type macrophages [48]. Collectively, Th2 cells are generally considered to have pro-tumor effects but may also have some anti-tumor activity.
5. Th17 Cell
Th17 cells secrete the IL-17 family cytokines IL-17A, IL-17F, IL-21, IL-22, and granulocyte macrophage colony-stimulating factor (GM-CSF). Taken together, they play a critical role in immune responses against extracellular bacteria and fungi at mucosal tissues [49][50]. IL-17 is associated with the induction of pro-inflammatory immune responses by increasing the expression of other pro-inflammatory cytokines, chemokines, and chemical mediators in various types of cells [51][52]. Accordingly, Th17 cells have been shown to be involved in chronic inflammatory diseases such as psoriasis, rheumatoid arthritis, and multiple sclerosis [53][54]. Th17 cells have also been reported to infiltrate into tumor tissues of various cancers, and the infiltration of Th17 cells is associated with both better and poor clinical outcomes [55][56]. Thus, Th17 cells are now considered to have both pro-tumor and anti-tumor activities (Figure 2) [57].
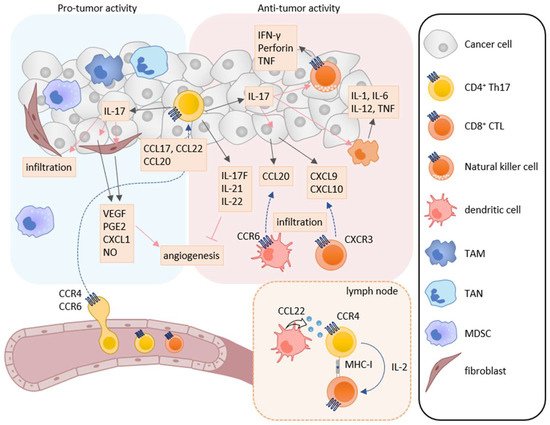
Figure 2. Roles of Th17 cells in tumor immunity. Th17 cells have both pro-tumor and anti-tumor activities in tumor immunity. IL-17A induces the production of angiogenic factors such as VEGF, PGE2, CXCL1, and NO from tumor cells and fibroblasts, leading to increase in angiogenesis. IL-17A also enhances the infiltration of MDSCs into tumor tissues. On the other hand, Th17 cells can directly induce tumor-specific CTLs via IL-2 production and MHC class I molecule expression in the lymph node. IL-17A also induces NK cells to express natural cytotoxicity receptor, perforin, TNF, and IFN-γ, and macrophages to express IL-1, IL-6, IL-12, and TNF. Furthermore, IL-17A induces the production of CXCL9 and CXCL10 in tumor cells and CCL20 in macrophages. These chemokines recruit CXCR3-expressing CTLs and CCR6-expressing immature DCs, respectively. In addition, IL-17F, IL-21, and IL-22 inhibit angiogenesis.
6. Treg Cell
Treg cells suppress immune responses under physiological and pathological conditions [58][59][60]. Numerous studies have shown that Treg cells inhibit the activation and proliferation of effector T cells and DCs, and play the major role in tumor escape from host immunosurveillance [58][59][60]. In the tumor microenvironment, infiltrated Treg cells secrete several immunosuppressive cytokines such as IL-10, TGF-β, and IL-35, which suppress the induction and activation of tumor-specific effector T cells (Figure 3) [59][60]. Furthermore, IL-10 and IL-35 derived from Treg cells promote the exhaustion of CD8+ tumor-infiltrating lymphocytes [61]. Treg cells also express cytotoxic T-lymphocyte antigen-4 (CTLA-4), a T cell inhibitory molecule, on their cell surface. CTLA-4 binds to costimulatory molecules CD80 and CD86 on DCs and thus blocks their binding to CD28 on T cells, resulting in inhibition of the costimulatory signals necessary for the induction of tumor-specific T cell responses [62]. Treg cells also express lymphocyte activation gene 3 (LAG-3), which inhibits the induction of tumor-specific T cell responses by suppressing DC activation via interaction with MHC class II [63][64]. Recently, cell metabolism has also been reported to be involved in the immunosuppressive mechanisms of Treg cells. While apoptotic Treg cells are a major source of ATP in the tumor microenvironment [65], Treg cells also express CD39 and CD73 and thus generate a large amount of adenosine from ATP in the tumor microenvironment, which inhibits the function and proliferation of effector T cells via the adenosine A2A receptor [66]. Treg cells also enhance the differentiation and immunosuppressive functions of MDSCs via the production of TGF-β [67]. In turn, MDSCs enhance the proliferation of Treg cells in the draining lymph nodes via the production of TGF-β [68].
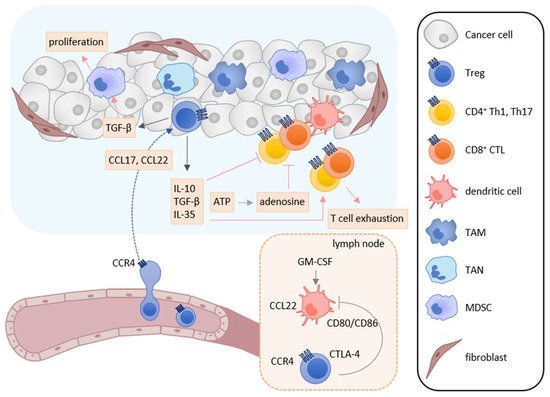
Figure 3. Roles of Treg cells in tumor immunity. In the tumor microenvironment, TAMs, TANs, tumor cells, and cancer-associated fibroblasts secrete CCL17 and/or CCL22, and recruit CCR4-expressing Treg cells into tumor tissues. Infiltrated Treg cells secret immunosuppressive cytokines such as IL-10, TGF-β, and IL-35. These cytokines suppress the activation and induction of tumor-specific T cell responses and induce T cell exhaustion. TGF-β also enhances the differentiation and immunosuppressive function of MDSCs. Furthermore, adenosine is generated from ATP derived from apoptotic Treg cells and inhibits the function and proliferation of effector T cells. Treg cells also inhibit the costimulatory signals of DCs to induce tumor-specific T cell responses by the CTLA-4-CD80/CD86 interaction.
7. TAM, MDSC, and TAN
TAMs are known to be abundantly present in the tumor microenvironment [6][7]. Many studies have shown that TAMs have a multiple role in tumor progression. TAMs enhance immunosuppression, tumor cell growth, metastasis, and angiogenesis by inducing the expression of cytokines, chemokines, and growth factors [69][70]. Consistently, TAM infiltration into the tumor microenvironment correlates with poor prognosis in most solid cancers [71][72]. Although TAMs express various chemokine receptors, the CCL2-CCR2 axis plays a dominant role in their recruitment into tumor tissues [10][73]. In addition, TAMs also utilize CCR5 and CXCR4 for their recruitment in some types of cancer [10][73]. MDSCs are also abundantly present in the tumor microenvironment [6][7]. MDSCs are a heterogenous population of immune cells and can be broadly divided into two categories: monocytic-MDSCs and polymorphonuclear-MDSCs [6][7]. Both MDSCs contribute to tumor progression by enhancing immunosuppression, angiogenesis, and epithelial–mesenchymal transition [6][7]. Monocytic-MDSCs indirectly and nonspecifically inhibit the activity of many types of effector cells by producing immunosuppressive mediators such as reactive nitrogen species, inducible nitric oxide synthase and arginase [74][75]. Furthermore, infiltrated monocytic-MDSCs have been shown to be able to differentiate into TAMs in the tumor microenvironment [74][75]. On the other hand, polymorphonuclear-MDSCs directly interact with CD8+ T cells and inhibit antigen-specific CD8+ T cell responses by producing reactive oxygen species in the draining lymph nodes [74][75]. Monocytic-MDSCs and polymorphonuclear-MDSCs utilize similar chemokine receptors as monocytes and neutrophils, respectively. Monocytic-MDSCs express CCR2 and CCR1/CCR5, and can be recruited into tumor tissues by CC chemokines such as CCL2 and CCL5, respectively [76][77]. On the other hand, polymorphonuclear-MDSCs express CXCR2 and can be recruited into tumor tissues by CXC chemokines such as CXCL8 [78][74][79]. MDSCs also express CXCR4 and can be recruited into tumor tissues by CXCL12 [78][74][79]. TANs have the same developmental origin and similar phenotypical features as polymorphonuclear-MDSCs [80][81]. TANs have pro-tumor activity by producing immunosuppressive soluble mediators such as TGF-β and arginase [80][81]. Similar to normal neutrophils, TANs utilize the CXCR2 axis for their recruitment [82]. In addition, TAMs, monocytic-MDSCs, polymorphonuclear-MDSCs, and TANs can produce the CCR4 ligands CCL17 and CCL22 in tumor tissues, contributing to tumor progression by recruiting CCR4-expressing Treg cells [3].
8. The Chemokine Superfamily as Therapeutic Targets in Cancer Immunotherapy
Although the chemokine superfamily have long been regarded as highly promising therapeutic targets for drug development, currently only three drugs are approved: Maraviroc, a small-molecule CCR5 antagonist for blocking infection by CCR5-tropic HIV-1; Plerixafor, a small-molecule CXCR4 antagonist for the mobilization of hematopoietic stem cells from the bone marrow for transplantation in patients with non-Hodgkin’s lymphoma and multiple myeloma; Mogamulizumab, a fully humanized and glyco-engineered monoclonal anti-CCR4 antibody for patients with aggressive/refractory adult T cell leukemia/lymphoma (ATLL) and cutaneous T cell lymphomas (CTCLs) [41][83][84]. However, the recent impressive therapeutic success of immune checkpoint inhibitors in cancer immunotherapy have opened the possibility of clinical application of drugs targeting chemokines and chemokine receptors as an adjunct therapeutic drug for cancer immunotherapy or chemotherapy. Indeed, as summarized in a recent review, a number of preclinical studies have shown significant therapeutic effects of drugs targeting chemokine receptors in cancer immunotherapy of various murine cancer models, and some of these approaches are currently being tested in clinical trials [85][86].
References
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10.
- Lin, J.X.; Leonard, W.J. Fine-Tuning Cytokine Signals. Annu. Rev. Immunol. 2019, 37, 295–324.
- Ozga, A.J.; Chow, M.T.; Luster, A.D. Chemokines and the immune response to cancer. Immunity 2021, 54, 859–874.
- Zhu, X.; Zhu, J. CD4 T Helper Cell Subsets and Related Human Immunological Disorders. Int. J. Mol. Sci. 2020, 21, 8011.
- Chatzileontiadou, D.S.M.; Sloane, H.; Nguyen, A.T.; Gras, S.; Grant, E.J. The Many Faces of CD4(+) T Cells: Immunological and Structural Characteristics. Int. J. Mol. Sci. 2020, 22, 73.
- Szebeni, G.J.; Vizler, C.; Nagy, L.I.; Kitajka, K.; Puskas, L.G. Pro-Tumoral Inflammatory Myeloid Cells as Emerging Therapeutic Targets. Int. J. Mol. Sci. 2016, 17, 1958.
- Okla, K.; Wertel, I.; Polak, G.; Surowka, J.; Wawruszak, A.; Kotarski, J. Tumor-Associated Macrophages and Myeloid-Derived Suppressor Cells as Immunosuppressive Mechanism in Ovarian Cancer Patients: Progress and Challenges. Int. Rev. Immunol. 2016, 35, 372–385.
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437.
- Sarvaiya, P.J.; Guo, D.; Ulasov, I.; Gabikian, P.; Lesniak, M.S. Chemokines in tumor progression and metastasis. Oncotarget 2013, 4, 2171–2185.
- Do, H.T.T.; Lee, C.H.; Cho, J. Chemokines and their Receptors: Multifaceted Roles in Cancer Progression and Potential Value as Cancer Prognostic Markers. Cancers (Basel) 2020, 12, 287.
- Kastenmuller, W.; Brandes, M.; Wang, Z.; Herz, J.; Egen, J.G.; Germain, R.N. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T cell responses in the lymph node. Immunity 2013, 38, 502–513.
- Rapp, M.; Wintergerst, M.W.M.; Kunz, W.G.; Vetter, V.K.; Knott, M.M.L.; Lisowski, D.; Haubner, S.; Moder, S.; Thaler, R.; Eiber, S.; et al. CCL22 controls immunity by promoting regulatory T cell communication with dendritic cells in lymph nodes. J. Exp. Med. 2019, 216, 1170–1181.
- Matsuo, K.; Itoh, T.; Koyama, A.; Imamura, R.; Kawai, S.; Nishiwaki, K.; Oiso, N.; Kawada, A.; Yoshie, O.; Nakayama, T. CCR4 is critically involved in effective antitumor immunity in mice bearing intradermal B16 melanoma. Cancer Lett. 2016, 378, 16–22.
- Matsuo, K.; Kitahata, K.; Kaibori, Y.; Arima, Y.; Iwama, A.; Ito, M.; Hara, Y.; Nagakubo, D.; Quan, Y.S.; Kamiyama, F.; et al. CCR4 Involvement in the Expansion of T Helper Type 17 Cells in a Mouse Model of Psoriasis. J. Investig. Dermatol. 2021, 141, 1985–1994.
- Schumann, K.; Lammermann, T.; Bruckner, M.; Legler, D.F.; Polleux, J.; Spatz, J.P.; Schuler, G.; Forster, R.; Lutz, M.B.; Sorokin, L.; et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 2010, 32, 703–713.
- Katou, F.; Ohtani, H.; Nakayama, T.; Nagura, H.; Yoshie, O.; Motegi, K. Differential expression of CCL19 by DC-Lamp+ mature dendritic cells in human lymph node versus chronically inflamed skin. J. Pathol. 2003, 199, 98–106.
- Johnson, L.A.; Jackson, D.G. Control of dendritic cell trafficking in lymphatics by chemokines. Angiogenesis 2014, 17, 335–345.
- Willimann, K.; Legler, D.F.; Loetscher, M.; Roos, R.S.; Delgado, M.B.; Clark-Lewis, I.; Baggiolini, M.; Moser, B. The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur. J. Immunol. 1998, 28, 2025–2034.
- Luther, S.A.; Tang, H.L.; Hyman, P.L.; Farr, A.G.; Cyster, J.G. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl. Acad. Sci. USA 2000, 97, 12694–12699.
- Baekkevold, E.S.; Yamanaka, T.; Palframan, R.T.; Carlsen, H.S.; Reinholt, F.P.; von Andrian, U.H.; Brandtzaeg, P.; Haraldsen, G. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J. Exp. Med. 2001, 193, 1105–1112.
- Sallusto, F.; Lanzavecchia, A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol. Rev. 2000, 177, 134–140.
- Matsuo, K.; Yoshie, O.; Kitahata, K.; Kamei, M.; Hara, Y.; Nakayama, T. Recent Progress in Dendritic Cell-Based Cancer Immunotherapy. Cancers (Basel) 2021, 13, 2495.
- Mosmann, T.R.; Coffman, R.L. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989, 7, 145–173.
- Murphy, K.M.; Ouyang, W.; Farrar, J.D.; Yang, J.; Ranganath, S.; Asnagli, H.; Afkarian, M.; Murphy, T.L. Signaling and transcription in T helper development. Annu. Rev. Immunol. 2000, 18, 451–494.
- Alspach, E.; Lussier, D.M.; Schreiber, R.D. Interferon gamma and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity. Cold Spring Harb. Perspect. Biol. 2019, 11, a028480.
- Mortara, L.; Balza, E.; Bruno, A.; Poggi, A.; Orecchia, P.; Carnemolla, B. Anti-cancer Therapies Employing IL-2 Cytokine Tumor Targeting: Contribution of Innate, Adaptive and Immunosuppressive Cells in the Anti-tumor Efficacy. Front. Immunol. 2018, 9, 2905.
- Wallin, R.P.; Screpanti, V.; Michaelsson, J.; Grandien, A.; Ljunggren, H.G. Regulation of perforin-independent NK cell-mediated cytotoxicity. Eur. J. Immunol. 2003, 33, 2727–2735.
- Corgnac, S.; Boutet, M.; Kfoury, M.; Naltet, C.; Mami-Chouaib, F. The Emerging Role of CD8(+) Tissue Resident Memory T (TRM) Cells in Antitumor Immunity: A Unique Functional Contribution of the CD103 Integrin. Front. Immunol. 2018, 9, 1904.
- Nersesian, S.; Schwartz, S.L.; Grantham, S.R.; MacLean, L.K.; Lee, S.N.; Pugh-Toole, M.; Boudreau, J.E. NK cell infiltration is associated with improved overall survival in solid cancers: A systematic review and meta-analysis. Transl. Oncol. 2021, 14, 100930.
- St Paul, M.; Ohashi, P.S. The Roles of CD8(+) T Cell Subsets in Antitumor Immunity. Trends Cell Biol. 2020, 30, 695–704.
- Kemp, R.A.; Ronchese, F. Tumor-specific Tc1, but not Tc2, cells deliver protective antitumor immunity. J. Immunol. 2001, 167, 6497–6502.
- Visekruna, A.; Ritter, J.; Scholz, T.; Campos, L.; Guralnik, A.; Poncette, L.; Raifer, H.; Hagner, S.; Garn, H.; Staudt, V.; et al. Tc9 cells, a new subset of CD8(+) T cells, support Th2-mediated airway inflammation. Eur. J. Immunol. 2013, 43, 606–618.
- Yen, H.R.; Harris, T.J.; Wada, S.; Grosso, J.F.; Getnet, D.; Goldberg, M.V.; Liang, K.L.; Bruno, T.C.; Pyle, K.J.; Chan, S.L.; et al. Tc17 CD8 T cells: Functional plasticity and subset diversity. J. Immunol. 2009, 183, 7161–7168.
- St Paul, M.; Saibil, S.D.; Lien, S.C.; Han, S.; Sayad, A.; Mulder, D.T.; Garcia-Batres, C.R.; Elford, A.R.; Israni-Winger, K.; Robert-Tissot, C.; et al. IL6 Induces an IL22(+) CD8(+) T-cell Subset with Potent Antitumor Function. Cancer Immunol. Res. 2020, 8, 321–333.
- Lu, Y.; Hong, B.; Li, H.; Zheng, Y.; Zhang, M.; Wang, S.; Qian, J.; Yi, Q. Tumor-specific IL-9-producing CD8+ Tc9 cells are superior effector than type-I cytotoxic Tc1 cells for adoptive immunotherapy of cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 2265–2270.
- Huber, M.; Heink, S.; Grothe, H.; Guralnik, A.; Reinhard, K.; Elflein, K.; Hunig, T.; Mittrucker, H.W.; Brustle, A.; Kamradt, T.; et al. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur. J. Immunol. 2009, 39, 1716–1725.
- Nakayama, T.; Hirahara, K.; Onodera, A.; Endo, Y.; Hosokawa, H.; Shinoda, K.; Tumes, D.J.; Okamoto, Y. Th2 Cells in Health and Disease. Annu. Rev. Immunol. 2017, 35, 53–84.
- Morel, P.A.; Oriss, T.B. Crossregulation between Th1 and Th2 cells. Crit. Rev. Immunol. 1998, 18, 275–303.
- Bachelerie, F.; Ben-Baruch, A.; Burkhardt, A.M.; Combadiere, C.; Farber, J.M.; Graham, G.J.; Horuk, R.; Sparre-Ulrich, A.H.; Locati, M.; Luster, A.D.; et al. International Union of Basic and Clinical Pharmacology. . LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol. Rev. 2014, 66, 1–79.
- Zlotnik, A.; Yoshie, O. The chemokine superfamily revisited. Immunity 2012, 36, 705–716.
- Yoshie, O.; Matsushima, K. CCR4 and its ligands: From bench to bedside. Int. Immunol. 2015, 27, 11–20.
- Protti, M.P.; De Monte, L. Cross-talk within the tumor microenvironment mediates Th2-type inflammation in pancreatic cancer. Oncoimmunology 2012, 1, 89–91.
- Saravia, J.; Chapman, N.M.; Chi, H. Helper T cell differentiation. Cell. Mol. Immunol. 2019, 16, 634–643.
- Basu, A.; Ramamoorthi, G.; Albert, G.; Gallen, C.; Beyer, A.; Snyder, C.; Koski, G.; Disis, M.L.; Czerniecki, B.J.; Kodumudi, K. Differentiation and Regulation of TH Cells: A Balancing Act for Cancer Immunotherapy. Front. Immunol. 2021, 12, 669474.
- Li, Z.; Jiang, J.; Wang, Z.; Zhang, J.; Xiao, M.; Wang, C.; Lu, Y.; Qin, Z. Endogenous interleukin-4 promotes tumor development by increasing tumor cell resistance to apoptosis. Cancer Res. 2008, 68, 8687–8694.
- Lorvik, K.B.; Hammarstrom, C.; Fauskanger, M.; Haabeth, O.A.; Zangani, M.; Haraldsen, G.; Bogen, B.; Corthay, A. Adoptive Transfer of Tumor-Specific Th2 Cells Eradicates Tumors by Triggering an In Situ Inflammatory Immune Response. Cancer Res. 2016, 76, 6864–6876.
- Dillon, S.R.; Sprecher, C.; Hammond, A.; Bilsborough, J.; Rosenfeld-Franklin, M.; Presnell, S.R.; Haugen, H.S.; Maurer, M.; Harder, B.; Johnston, J.; et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 2004, 5, 752–760.
- Kan, T.; Feldman, E.; Timaner, M.; Raviv, Z.; Shen-Orr, S.; Aronheim, A.; Shaked, Y. IL-31 induces antitumor immunity in breast carcinoma. J. Immunother. Cancer 2020, 8, e001010.
- Bedoya, S.K.; Lam, B.; Lau, K.; Larkin, J., 3rd. Th17 cells in immunity and autoimmunity. Clin. Dev. Immunol. 2013, 2013, 986789.
- Knochelmann, H.M.; Dwyer, C.J.; Bailey, S.R.; Amaya, S.M.; Elston, D.M.; Mazza-McCrann, J.M.; Paulos, C.M. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell. Mol. Immunol 2018, 15, 458–469.
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133–1141.
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132.
- Brembilla, N.C.; Senra, L.; Boehncke, W.H. The IL-17 Family of Cytokines in Psoriasis: IL-17A and Beyond. Front. Immunol. 2018, 9, 1682.
- Dardalhon, V.; Korn, T.; Kuchroo, V.K.; Anderson, A.C. Role of Th1 and Th17 cells in organ-specific autoimmunity. J. Autoimmun. 2008, 31, 252–256.
- Punt, S.; Langenhoff, J.M.; Putter, H.; Fleuren, G.J.; Gorter, A.; Jordanova, E.S. The correlations between IL-17 vs. Th17 cells and cancer patient survival: A systematic review. Oncoimmunology 2015, 4, e984547.
- Bailey, S.R.; Nelson, M.H.; Himes, R.A.; Li, Z.; Mehrotra, S.; Paulos, C.M. Th17 cells in cancer: The ultimate identity crisis. Front. Immunol. 2014, 5, 276.
- Zou, W.; Restifo, N.P. T(H)17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol. 2010, 10, 248–256.
- Josefowicz, S.Z.; Lu, L.F.; Rudensky, A.Y. Regulatory T cells: Mechanisms of differentiation and function. Annu. Rev. Immunol. 2012, 30, 531–564.
- Tanaka, A.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017, 27, 109–118.
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 2020, 19, 116.
- Sawant, D.V.; Yano, H.; Chikina, M.; Zhang, Q.; Liao, M.; Liu, C.; Callahan, D.J.; Sun, Z.; Sun, T.; Tabib, T.; et al. Adaptive plasticity of IL-10(+) and IL-35(+) Treg cells cooperatively promotes tumor T cell exhaustion. Nat. Immunol. 2019, 20, 724–735.
- Walker, L.S.; Sansom, D.M. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 2011, 11, 852–863.
- Liang, B.; Workman, C.; Lee, J.; Chew, C.; Dale, B.M.; Colonna, L.; Flores, M.; Li, N.; Schweighoffer, E.; Greenberg, S.; et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J. Immunol. 2008, 180, 5916–5926.
- Maruhashi, T.; Okazaki, I.M.; Sugiura, D.; Takahashi, S.; Maeda, T.K.; Shimizu, K.; Okazaki, T. LAG-3 inhibits the activation of CD4(+) T cells that recognize stable pMHCII through its conformation-dependent recognition of pMHCII. Nat. Immunol. 2018, 19, 1415–1426.
- Ohta, A.; Sitkovsky, M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 2001, 414, 916–920.
- Ohta, A.; Kini, R.; Ohta, A.; Subramanian, M.; Madasu, M.; Sitkovsky, M. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front. Immunol. 2012, 3, 190.
- Lee, C.R.; Kwak, Y.; Yang, T.; Han, J.H.; Park, S.H.; Ye, M.B.; Lee, W.; Sim, K.Y.; Kang, J.A.; Kim, Y.C.; et al. Myeloid-Derived Suppressor Cells Are Controlled by Regulatory T Cells via TGF-beta during Murine Colitis. Cell Rep. 2016, 17, 3219–3232.
- Ghiringhelli, F.; Puig, P.E.; Roux, S.; Parcellier, A.; Schmitt, E.; Solary, E.; Kroemer, G.; Martin, F.; Chauffert, B.; Zitvogel, L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med. 2005, 202, 919–929.
- De Vlaeminck, Y.; Gonzalez-Rascon, A.; Goyvaerts, C.; Breckpot, K. Cancer-Associated Myeloid Regulatory Cells. Front. Immunol. 2016, 7, 113.
- Malekghasemi, S.; Majidi, J.; Baghbanzadeh, A.; Abdolalizadeh, J.; Baradaran, B.; Aghebati-Maleki, L. Tumor-Associated Macrophages: Protumoral Macrophages in Inflammatory Tumor Microenvironment. Adv. Pharm. Bull. 2020, 10, 556–565.
- Heusinkveld, M.; van der Burg, S.H. Identification and manipulation of tumor associated macrophages in human cancers. J. Transl. Med. 2011, 9, 216.
- Bingle, L.; Brown, N.J.; Lewis, C.E. The role of tumour-associated macrophages in tumour progression: Implications for new anticancer therapies. J. Pathol. 2002, 196, 254–265.
- Argyle, D.; Kitamura, T. Targeting Macrophage-Recruiting Chemokines as a Novel Therapeutic Strategy to Prevent the Progression of Solid Tumors. Front. Immunol. 2018, 9, 2629.
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220.
- Youn, J.I.; Gabrilovich, D.I. The biology of myeloid-derived suppressor cells: The blessing and the curse of morphological and functional heterogeneity. Eur. J. Immunol. 2010, 40, 2969–2975.
- Murdoch, C.; Giannoudis, A.; Lewis, C.E. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood 2004, 104, 2224–2234.
- Qian, B.Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225.
- OuYang, L.Y.; Wu, X.J.; Ye, S.B.; Zhang, R.X.; Li, Z.L.; Liao, W.; Pan, Z.Z.; Zheng, L.M.; Zhang, X.S.; Wang, Z.; et al. Tumor-induced myeloid-derived suppressor cells promote tumor progression through oxidative metabolism in human colorectal cancer. J. Transl. Med. 2015, 13, 47.
- Toh, B.; Wang, X.; Keeble, J.; Sim, W.J.; Khoo, K.; Wong, W.C.; Kato, M.; Prevost-Blondel, A.; Thiery, J.P.; Abastado, J.P. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol. 2011, 9, e1001162.
- Karin, N. The Development and Homing of Myeloid-Derived Suppressor Cells: From a Two-Stage Model to a Multistep Narrative. Front. Immunol. 2020, 11, 557586.
- Lecot, P.; Sarabi, M.; Pereira Abrantes, M.; Mussard, J.; Koenderman, L.; Caux, C.; Bendriss-Vermare, N.; Michallet, M.C. Neutrophil Heterogeneity in Cancer: From Biology to Therapies. Front. Immunol. 2019, 10, 2155.
- Cheng, Y.; Mo, F.; Li, Q.; Han, X.; Shi, H.; Chen, S.; Wei, Y.; Wei, X. Targeting CXCR2 inhibits the progression of lung cancer and promotes therapeutic effect of cisplatin. Mol. Cancer 2021, 20, 62.
- Yoshie, O. CCR4 as a Therapeutic Target for Cancer Immunotherapy. Cancers (Basel) 2021, 13, 5542.
- Miao, M.; De Clercq, E.; Li, G. Clinical significance of chemokine receptor antagonists. Expert Opin. Drug Metab. Toxicol. 2020, 16, 11–30.
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572.
- Mollica Poeta, V.; Massara, M.; Capucetti, A.; Bonecchi, R. Chemokines and Chemokine Receptors: New Targets for Cancer Immunotherapy. Front. Immunol. 2019, 10, 379.
More
Information
Subjects:
Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
707
Revisions:
2 times
(View History)
Update Date:
24 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




