Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Various immune cells are involved in host immune responses to cancer. T-helper (Th) 1 cells, cytotoxic CD8+ T cells, and natural killer cells are the major effector cells in anti-tumor immunity, whereas cells such as regulatory T cells and myeloid-derived suppressor cells are negatively involved in anti-tumor immunity. Th2 cells and Th17 cells have been shown to have both pro-tumor and anti-tumor activities. The migratory properties of various immune cells are essential for their function and critically regulated by the chemokine superfamily.
- chemokine
- chemokine receptor
- Th1
- Th2
- Th17
- regulatory T
- macrophage
- neutrophil
- tumor microenvironment
1. Introduction
Tumor immunity is initiated by the recognition of tumor antigens by the immune system [1]. Antigen priming and effector cell differentiation are regulated by complex processes involving various cell populations, cytokines, and chemokines [1,2,3]. While CD4+ helper T cells are known to orchestrate immune responses, CD4+ T cells are heterogenous and composed of various functional subsets including T-helper (Th) 1 cells, Th2 cells, Th17 cells, and regulatory T (Treg) cells [4,5]. These T cell subsets are characterized by the secretion of signature cytokines and differentially involved in tumor immunity [4,5]. Furthermore, CD8+ cytotoxic lymphocytes (CTLs) and natural killer (NK) cells play direct roles in the elimination of tumor cells. Tumor tissues are also highly enriched with innate immune cells such as myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), and tumor-associated neutrophils (TANs) [6,7]. These various immune cells are known to express characteristic chemokine receptors and are elaborately regulated in their migration and tissue localization by respective chemokine ligands [8,9,10]. Accordingly, some chemokines and their receptors may be involved in the elimination of tumor cells by recruiting anti-tumor effector cells, whereas others may promote tumor progression by attracting immunosuppressive cells. In addition, some chemokines and their receptors are known to play critical roles in the interactions of dendric cells (DCs) and T cells [11,12,13,14]. Thus, the chemokine superfamily has a multifaceted role in host tumor immunity.
2. Induction of Tumor-Specific T Cell Responses
T cell-mediated anti-tumor immunity is thought to be achieved by a multistep process called the cancer-immunity cycle [1]. It includes the following seven steps: (1) release of cancer antigens, (2) cancer antigen presentation, (3) priming and activation, (4) trafficking of T cells to tumors, (5) infiltration of T cells into tumors, (6) recognition of cancer cells by T cells, and (7) killing of cancer cells. Thus, the cycle is initiated by the uptake of tumor antigens, including tumor-associated antigens and neoantigens, by DCs, the professional antigen-presenting cells. Tumor antigen-captured DCs then migrate into the draining lymph nodes where recirculating naïve T cells and memory T cells scan antigenic peptides presented by DCs in association with class I and class II major histocompatibility complex (MHC) molecules (Figure 1). The CCL19/CCL21-CCR7 axis is known to play a pivotal role in the migratory activities of DCs and recirculating T cells [1,15]. While immature DCs in peripheral tissues dominantly express CCR6, the surface expression of CCR7 is upregulated upon antigen-loading and DC maturation [1,15]. Since CCL21 is abundantly produced by lymphatic vessels, CCR7-expressing DCs initiate trans-lymphatic migration and home into T cell areas of the draining lymph nodes where matured DCs start producing CCL19 (Figure 1) [3,16,17]. The lymph nodes also have unique vascular structures called high endothelial venules (HEVs), which produce CCL21 and function as the gateways for recirculating naïve T cells and memory T cells that commonly express CCR7 (Figure 1) [18,19]. In addition, although CCL19 is not produced by HEVs, it is displayed on the luminal surfaces of HEVs by transcytosis [20]. After homing into the lymph nodes, naïve T cells and memory T cells further migrate toward CCL19-producing mature DCs localized in the T cell areas [1,15]. Upon encounter with cognate antigenic peptides presented by mature DCs, antigen-specific naïve T cells proliferate and differentiate into various effector T cell subsets in accordance with the local cytokine milieu, whereas memory T cells start rapid expansions for recall immune responses [21]. Furthermore, conventional DCs have two subtypes known as type 1 (cDC1s) and type 2 (cDC2s) [22]. It is now known that cDC1s preferentially induce the differentiation of naïve CD4+ T cells and CD8+ T cells into Th1 cells and CTLs, respectively [3,16,17], whereas cDC2s preferentially induce the differentiation of naïve CD4+ T cells into Th2 cells and Th17 cells (Figure 1) [3,16,17]. Importantly, cDC1s selectively express XCR1 and are the most efficient DCs in the cross-presentation of exogenous antigens to CD8+ T cells [22]. Of note, while cDC1s activate CCR7-expressing naïve CD8+ T cells [22], activated CD8+ T cells in turn produce XCL1, the ligand of XCR1 (Figure 1) [22]. This further promotes the interactions of CD8+ T cells and cDC1s, leading to full differentiation of effector CTLs [22]. In secondary immune responses, mature DCs also produce CCL3, CCL4, CCL5, CCL17, CCL22, CXCL9, CXCL10, and CXCL11 [22]. Since Th1 cells express CCR5 and CXCR3, while Th2 cells, Th17 cells, and Treg cells express CCR4 [22], these chemokine–chemokine receptor axes contribute to the rapid expansion of effector T cells (Figure 1).
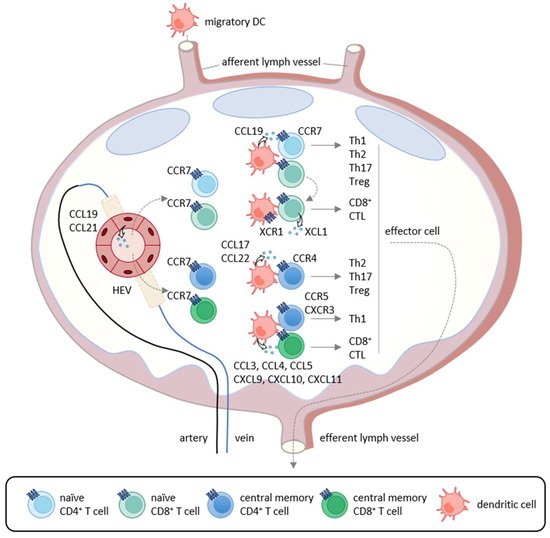
Figure 1. Chemokine-mediated T cell immune responses in the lymph node. In primary immune responses, antigen-captured DCs migrate into the draining lymph nodes via afferent lymphatics using the CCL19/CCL21-CCR7 axis. In the lymph nodes, mature DCs produce CCL19 and interact with recirculating CCR7-expressing naïve CD4+ T cells that home into the lymph nodes via the high endothelial venules (HEVs). If stimulated by cognate antigenic peptides presented by mature DCs, naïve T cells differentiate into Th1 cells, Th2 cells, Th17 cells, and Treg cells according to the local cytokine milieu.
3. Th1 Cell, CTL, and NK Cell
Th1 cells are involved in cellular immunity by secreting Th1-type cytokines such as interferon (IFN)-γ, Interleukin (IL)-2 and tumor necrosis factor (TNF)-α [23,24]. Th1-type cytokines promote the differentiation of naïve CD8+ T cells into CTLs [23,24] and also enhance the cytotoxic activity of CD8+ T cells and NK cells [25,26]. CD8+ T cells and NK cells are able to directly eliminate tumor cells by secreting cytotoxic molecules such as perforin and granzyme. NK cells are also able to induce apoptosis in tumor cells via TNF family molecules such as FAS ligand (FASL) and TNF-related apoptosis-inducing ligand (TRAIL) [27]. Thus, Th1 cells, CD8+ T cells, and NK cells are the major effector cells in anti-tumor immunity. Indeed, infiltration of CD8+ T cells and NK cells correlates with better clinical outcomes and therapeutic responses in various types of cancer [28,29]. In addition, recent studies have revealed the existence of CD8+ T cell subsets such as IFN-γ-expressing Tc1 cells, IL-4-expressing Tc2 cells, IL-9-expressing Tc9 cells, IL-17-expressing Tc17 cells, and IL-22-expressing Tc22 cells [30,31,32,33,34]. Tc1, Tc2, and Tc22 cells produce high levels of perforin and granzyme and have high cytotoxic activity, while Tc9 and Tc17 produce low levels of cytotoxic molecules and have poor cytotoxic activity [35,36]. The respective roles of these various Tc subsets in tumor immunity remain to be seen.
4. Th2 Cell
Th2 cells are involved in humoral immunity by secreting Th2-type cytokines, such as IL-4, IL-5, IL-10, and IL-13 [91,92]. Th2 cells are shown to express CCR4, CCR8, and, to a lesser extent, CCR3 [46,85,93]. It has been reported that the expression levels of respective chemokine ligands are upregulated in some types of cancer, and Th2 cells are increased in tumor tissues and the draining lymph nodes [94]. Th2 cytokines inhibit the differentiation and function of Th1 cells, while Th1 cytokines inhibit the differentiation and function of Th2 cells [95]. Thus, Th2 cells may indirectly suppress anti-tumor immunity by negatively regulating Th1 responses. In particular, IL-10 is known to suppress Th1-mediated immune responses [96]. IL-4 has also been reported to enhance tumor growth by inhibiting apoptosis in a murine fibrosarcoma model [97]. On the other hand, it has also been demonstrated that adoptive transfer of tumor-specific Th2 cells protected mice against lethal challenge of murine myeloma and lymphoma through the induction of Th2-type inflammation in tumor tissues. However, Th2-mediated anti-tumor effects did not require B cells, NKT cells or CD8+ T cells and the mechanism was unknown [98]. Of note, Th2 cells are also known to secrete IL-31 [99]. Recently, it has been reported that IL-31-overexpressing murine breast carcinoma shows reduced tumor growth by inhibiting the activity of immunosuppressive cells such as Treg cells, MDSCs, and M2-type macrophages [100]. Collectively, Th2 cells are generally considered to have pro-tumor effects but may also have some anti-tumor activity.
5. Th17 Cell
Th17 cells secrete the IL-17 family cytokines IL-17A, IL-17F, IL-21, IL-22, and granulocyte macrophage colony-stimulating factor (GM-CSF). Taken together, they play a critical role in immune responses against extracellular bacteria and fungi at mucosal tissues [101,102]. IL-17 is associated with the induction of pro-inflammatory immune responses by increasing the expression of other pro-inflammatory cytokines, chemokines, and chemical mediators in various types of cells [103,104]. Accordingly, Th17 cells have been shown to be involved in chronic inflammatory diseases such as psoriasis, rheumatoid arthritis, and multiple sclerosis [105,106]. Th17 cells have also been reported to infiltrate into tumor tissues of various cancers, and the infiltration of Th17 cells is associated with both better and poor clinical outcomes [107,108]. Thus, Th17 cells are now considered to have both pro-tumor and anti-tumor activities (Figure 2) [109].
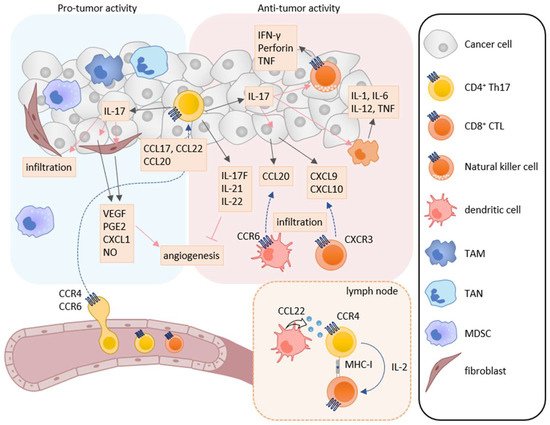
Figure 2. Roles of Th17 cells in tumor immunity. Th17 cells have both pro-tumor and anti-tumor activities in tumor immunity. IL-17A induces the production of angiogenic factors such as VEGF, PGE2, CXCL1, and NO from tumor cells and fibroblasts, leading to increase in angiogenesis. IL-17A also enhances the infiltration of MDSCs into tumor tissues. On the other hand, Th17 cells can directly induce tumor-specific CTLs via IL-2 production and MHC class I molecule expression in the lymph node. IL-17A also induces NK cells to express natural cytotoxicity receptor, perforin, TNF, and IFN-γ, and macrophages to express IL-1, IL-6, IL-12, and TNF. Furthermore, IL-17A induces the production of CXCL9 and CXCL10 in tumor cells and CCL20 in macrophages. These chemokines recruit CXCR3-expressing CTLs and CCR6-expressing immature DCs, respectively. In addition, IL-17F, IL-21, and IL-22 inhibit angiogenesis.
6. Treg Cell
Treg cells suppress immune responses under physiological and pathological conditions [151,152,153]. Numerous studies have shown that Treg cells inhibit the activation and proliferation of effector T cells and DCs, and play the major role in tumor escape from host immunosurveillance [151,152,153]. In the tumor microenvironment, infiltrated Treg cells secrete several immunosuppressive cytokines such as IL-10, TGF-β, and IL-35, which suppress the induction and activation of tumor-specific effector T cells (Figure 3) [152,153]. Furthermore, IL-10 and IL-35 derived from Treg cells promote the exhaustion of CD8+ tumor-infiltrating lymphocytes [154]. Treg cells also express cytotoxic T-lymphocyte antigen-4 (CTLA-4), a T cell inhibitory molecule, on their cell surface. CTLA-4 binds to costimulatory molecules CD80 and CD86 on DCs and thus blocks their binding to CD28 on T cells, resulting in inhibition of the costimulatory signals necessary for the induction of tumor-specific T cell responses [155]. Treg cells also express lymphocyte activation gene 3 (LAG-3), which inhibits the induction of tumor-specific T cell responses by suppressing DC activation via interaction with MHC class II [156,157]. Recently, cell metabolism has also been reported to be involved in the immunosuppressive mechanisms of Treg cells. While apoptotic Treg cells are a major source of ATP in the tumor microenvironment [158], Treg cells also express CD39 and CD73 and thus generate a large amount of adenosine from ATP in the tumor microenvironment, which inhibits the function and proliferation of effector T cells via the adenosine A2A receptor [125]. Treg cells also enhance the differentiation and immunosuppressive functions of MDSCs via the production of TGF-β [159]. In turn, MDSCs enhance the proliferation of Treg cells in the draining lymph nodes via the production of TGF-β [160].
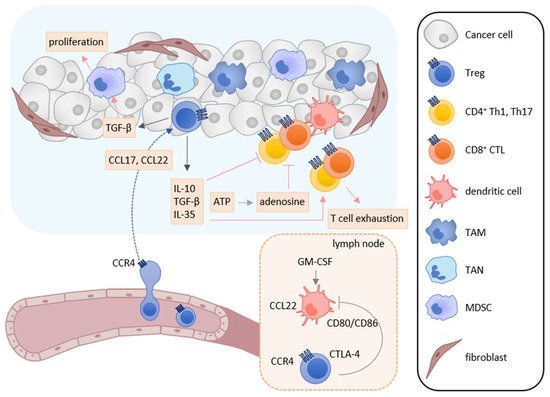
Figure 3. Roles of Treg cells in tumor immunity. In the tumor microenvironment, TAMs, TANs, tumor cells, and cancer-associated fibroblasts secrete CCL17 and/or CCL22, and recruit CCR4-expressing Treg cells into tumor tissues. Infiltrated Treg cells secret immunosuppressive cytokines such as IL-10, TGF-β, and IL-35. These cytokines suppress the activation and induction of tumor-specific T cell responses and induce T cell exhaustion. TGF-β also enhances the differentiation and immunosuppressive function of MDSCs. Furthermore, adenosine is generated from ATP derived from apoptotic Treg cells and inhibits the function and proliferation of effector T cells. Treg cells also inhibit the costimulatory signals of DCs to induce tumor-specific T cell responses by the CTLA-4-CD80/CD86 interaction.
7. TAM, MDSC, and TAN
TAMs are known to be abundantly present in the tumor microenvironment [6,7]. Many studies have shown that TAMs have a multiple role in tumor progression. TAMs enhance immunosuppression, tumor cell growth, metastasis, and angiogenesis by inducing the expression of cytokines, chemokines, and growth factors [186,187]. Consistently, TAM infiltration into the tumor microenvironment correlates with poor prognosis in most solid cancers [188,189]. Although TAMs express various chemokine receptors, the CCL2-CCR2 axis plays a dominant role in their recruitment into tumor tissues [10,52]. In addition, TAMs also utilize CCR5 and CXCR4 for their recruitment in some types of cancer [10,52]. MDSCs are also abundantly present in the tumor microenvironment [6,7]. MDSCs are a heterogenous population of immune cells and can be broadly divided into two categories: monocytic-MDSCs and polymorphonuclear-MDSCs [6,7]. Both MDSCs contribute to tumor progression by enhancing immunosuppression, angiogenesis, and epithelial–mesenchymal transition [6,7]. Monocytic-MDSCs indirectly and nonspecifically inhibit the activity of many types of effector cells by producing immunosuppressive mediators such as reactive nitrogen species, inducible nitric oxide synthase and arginase [190,191]. Furthermore, infiltrated monocytic-MDSCs have been shown to be able to differentiate into TAMs in the tumor microenvironment [190,191]. On the other hand, polymorphonuclear-MDSCs directly interact with CD8+ T cells and inhibit antigen-specific CD8+ T cell responses by producing reactive oxygen species in the draining lymph nodes [190,191]. Monocytic-MDSCs and polymorphonuclear-MDSCs utilize similar chemokine receptors as monocytes and neutrophils, respectively. Monocytic-MDSCs express CCR2 and CCR1/CCR5, and can be recruited into tumor tissues by CC chemokines such as CCL2 and CCL5, respectively [79,192]. On the other hand, polymorphonuclear-MDSCs express CXCR2 and can be recruited into tumor tissues by CXC chemokines such as CXCL8 [70,190,193]. MDSCs also express CXCR4 and can be recruited into tumor tissues by CXCL12 [70,190,193]. TANs have the same developmental origin and similar phenotypical features as polymorphonuclear-MDSCs [194,195]. TANs have pro-tumor activity by producing immunosuppressive soluble mediators such as TGF-β and arginase [194,195]. Similar to normal neutrophils, TANs utilize the CXCR2 axis for their recruitment [196]. In addition, TAMs, monocytic-MDSCs, polymorphonuclear-MDSCs, and TANs can produce the CCR4 ligands CCL17 and CCL22 in tumor tissues, contributing to tumor progression by recruiting CCR4-expressing Treg cells [3].
8. The Chemokine Superfamily as Therapeutic Targets in Cancer Immunotherapy
Although the chemokine superfamily have long been regarded as highly promising therapeutic targets for drug development, currently only three drugs are approved: Maraviroc, a small-molecule CCR5 antagonist for blocking infection by CCR5-tropic HIV-1; Plerixafor, a small-molecule CXCR4 antagonist for the mobilization of hematopoietic stem cells from the bone marrow for transplantation in patients with non-Hodgkin’s lymphoma and multiple myeloma; Mogamulizumab, a fully humanized and glyco-engineered monoclonal anti-CCR4 antibody for patients with aggressive/refractory adult T cell leukemia/lymphoma (ATLL) and cutaneous T cell lymphomas (CTCLs) [93,173,197]. However, the recent impressive therapeutic success of immune checkpoint inhibitors in cancer immunotherapy have opened the possibility of clinical application of drugs targeting chemokines and chemokine receptors as an adjunct therapeutic drug for cancer immunotherapy or chemotherapy. Indeed, as summarized in a recent review, a number of preclinical studies have shown significant therapeutic effects of drugs targeting chemokine receptors in cancer immunotherapy of various murine cancer models, and some of these approaches are currently being tested in clinical trials [198,199].
This entry is adapted from the peer-reviewed paper 10.3390/cancers13236132
This entry is offline, you can click here to edit this entry!
