Upland rivers across Europe still exhibit undisturbed conditions and represent a treasure that we cannot afford to lose. We hypothesize that the combination of pristine and modified conditions could demonstrate biological responses along the stressor gradients. Thus, the response of aquatic macrophyte communities to anthropogenic stressors along upland rivers in Bulgaria was studied. Six stressors were selected out of 36 parameters grouped into hydromorphological, chemical variables and combined drivers (catchment land use). The stressors strongly affected species richness on the basis of biological type (bryophytes vs. vascular plants) and ecomorphological type (hydrophytes vs. helophytes). Hydrological alteration expressed by the change of the river’s base flow and altered riparian habitats has led to a suppression of bryophytes and a dominance of riverbank plant communities. Seventy-five percent of mountain sites were lacking bryophytes, and the vegetation at semi-mountainous sites was dominated by vascular plants. It can be concluded that hydropeaking, organic and inorganic pollution, and discontinuous urban structures caused important modifications in the aquatic macrophyte assemblages. Macrophyte abundance and the biological and ecomorphological type of aquatic macrophytes reflect multi-stressor effects in upland rivers.
1. Introduction
“Good ecological status” within the context of the European Water Framework Directive is currently achieved by only 40% of European rivers
[1]. Approximately 40% of the highland and mid-altitude rivers in Europe are affected by hydromorphological pressures
[2]. There are numerous anthropogenic activities that contribute to the fact that approximately 60% of surface water bodies do not have a good ecological status. Among those with the most significant impact are agriculture, forestry and aquaculture, hydropower generation, and urban settlements
[3]. As for the stressors, almost half of the European water bodies are affected by two or more stressors
[3]. Nutrients expressed by nitrogen pollution, hydromorphology, and catchment land use were reported as major factors affecting the ecological status during the period 2004–2009
[4]. This trend has continued in recent years. Physical alterations affecting river hydromorphology and diffuse pollution are among the most widespread stressors simultaneously affecting Europe’s waters
[5]. The latest research on European rivers reported that almost all river types are more or less affected by riparian land use, hydrological changes, nutrient enrichment, and the input of toxic substances
[1].
The protection and restoration of freshwater ecosystems depend on the reliable detection of the stressors’ impact based on the aquatic biota. Most of the monitoring and assessment methods are linked to nutrient pollution, while only a few assess the impact of hydromorphological and multiple stressors
[5]. A recent review of papers dedicated to aquatic ecology revealed that nutrient stress was studied in 71% to 98% of multi-stress situations
[6]. Despite an increasing number of scientific investigations, there are still open questions regarding pathways between stressors, biota, and ecological status. Another important issue is the assessment of the effects of simultaneous stressors and their possible antagony, additivity, and synergy
[7].
Aquatic macrophytes have the ability to give a complex response to multiple stress factors and are recommended for the assessment of multiple stressors. Macrophyte-based metrics were found to be useful for assessing ecological quality at the river basin scale
[8]. Macrophytes showed significant correlations with habitat degradation and particularly with woody riparian vegetation
[9]. A strong correlation between macrophyte metrics, land-use gradient, and eutrophication/organic pollution in European mountain streams has been documented
[10]. Given this background, we collected a database of aquatic macrophytes in Bulgaria (southeastern Europe) and made an attempt to define the driving forces for their communities in upland rivers.
Rivers in mountain regions are under less severe impacts than lowland rivers
[2]. Nevertheless, land use in the mountains can alter river habitats, change sediment and nutrient regimes, and pose certain toxic risks. Moreover, a stronger dependence of aquatic communities on the surrounding terrestrial ecosystem is expected in upland rivers
[9]. In this study, we look into the stressors affecting mountain rivers and aquatic macrophyte communities as a tool to detect multi-stressor effects in these valuable aquatic ecosystems.
Bulgaria is characterized by its relief diversity and rich geological history. The Stara planina and Rila-Pirin-Rhodopes are the major mountain massifs in Bulgaria
[11]. The Balkan Mountains form a phytoclimatic barrier and divide the country into two ecoregions: 12 Pontic Province for northern and 7 Eastern Balkans for southern rivers
[12]. Almost all rivers in northern Bulgaria (Ecoregion 12) have their source in the Balkan Mountains
[13]. The Peri-Aegean drainage basin (Ecoregion 7) consists of the Struma, Mesta, and Maritsa river systems. The rivers, which flow directly into the Black Sea, belong to the Euxinian (Black Sea) drainage area and are usually short and small. The studied upland rivers belong to four altitude vegetation belts: xerothermic oak forests, xeromesophytic oak and hornbeam forests, beech belt, and coniferous belt
[14]. Point source pollution with untreated waste waters and diffuse pollution from agriculture were recognized as the most significant pressures on Bulgarian rivers
[15]. However, official monitoring data for Bulgarian rivers show significant gaps.
2. Studied Sites and Stressors
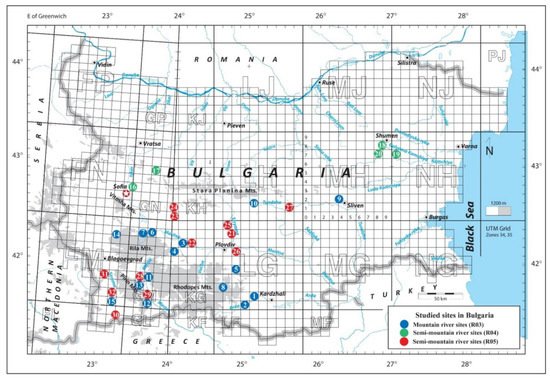
Thirty-two sites at mountain and semi-mountain rivers in Bulgaria were studied during the vegetation season in 2020 (Figure 1, Annex 1). Sites had mixed carbonate-siliceous bedrock and were located at considerably variable altitudes (between 71 and 1292 m a.s.l.). River water pH was neutral to slightly alkaline.
Figure 1. Location of the studied river sites. Refer to Annex 1 for the numbering and national types of the sites.
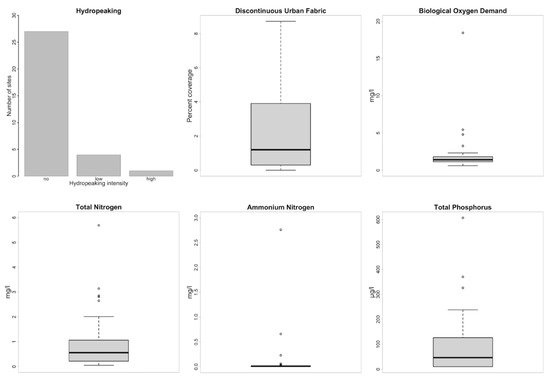
A summary of the measured stressors (n = 36) is given in Annex 2. The most relevant stressors were selected on the basis of their range in the dataset (Figure 2) and co-correlation with other stressors (Spearman correlation coefficient of Rs < 0.8).
Figure 2. Frequency and distribution of the six anthropogenic stressors analyzed in this study.
3. Aquatic Macrophyte Assemblage Patterns
Fifty-eight macrophyte species were recorded (Table 1); among them were one alga, five mosses, and 52 vascular plants. The most common species were the aquatic moss Platyhypnidium riparioides and the helophyte Mentha longifolia. Vascular plants had higher species richness than bryophytes (5 species). Among vascular plants, helophytes were the most numerous group (24 species). The number of taxa per river site varied between 1 and 13. Aquatic macrophytes had not been recorded at one site (site 10) due to difficult conditions for sampling during a flood event.
Table 1. List of species and their codes.
| Algae |
Code |
| Lemanea fluviatilis |
LEM.FLU |
| Mosses |
|
| Brachythecium rivulare Schimp. |
BRA.RIV |
| Cratoneuron filicinum (Hedw.) Spruce |
CRA.FIL |
| Fontinalis antipyretica Hedw. |
FON.ANT |
| Leptodictyum riparium (Hedw.) Warnst. |
LEP.RIP |
| Platyhypnidium riparioides (Hedw.) Dixon |
PLA.RIP |
| Vascular plants |
|
| Pteridophytes |
|
| Equisetum arvense L. |
EQU.ARV |
| Equisetum fluviatile L. |
EQU.FLU |
| Equisetum sylvaticum L. |
EQU.SYL |
| Equisetum telmateia Ehrh. |
EQU.TEL |
| Hydrophytes |
|
| Callitriche stagnalis Scop. |
CAL.STA |
| Ceratophyllum demersum L. |
CER.DEM |
| Ceratophyllum submersum L. |
CER.SUB |
| Elodea canadensis Michx. |
ELO.CAN |
| Lemna minor L. |
LEM.MIN |
| Myriophyllum spicatum L. |
MYR.SPI |
| Potamogeton berchtoldii Fieber |
POT.BER |
| Potamogeton crispus L. |
POT.CRI |
| Potamogeton nodosus Poir. |
POT.NOD |
| Potamogeton pectinatus L. |
POT.PEC |
| Potamogeton perfoliatus L. |
POT.PER |
| Potamogeton pusillus L. |
POT.PUS |
| Ranunculus trichophyllus Chaix |
RAN.TRI |
| Spirodela polyrhiza (L.) Schleid. |
SPI.POL |
| Hygrophytes |
|
| Bidens tripartitus L. |
BID.TRI |
| Cyperus longus L. |
CYP.LON |
| Epilobium hirsutum L. |
EPI.HIR |
| Myosoton aquaticum (L.) Moench |
MYO.AQU |
| Paspalum paspalodes (Michx.) Scribn. |
PAS.PAS |
| Petasites hybridus (L.) G. Gaertn. & al. |
PET.HYB |
| Polygonum lapathifolium L. |
POL.LAP |
| Polygonum mite Schrank |
POL.MIT |
| Ranunculus repens L. |
RAN.REP |
| Solanum dulcamara L. |
SOL.DUL |
| Helophytes |
|
| Alisma lanceolatum With. |
ALI.LAN |
| Alisma plantago-aquatica L. |
ALI.PLA |
| Berula erecta (Huds.) Coville |
BER.ERE |
| Cyperus fuscus L. |
CYP.FUS |
| Echinochloa crus-galli (L.) P. Beauv. |
ECH.CRU |
| Glyceria fluitans (L.) R. Br. |
GLY.FLU |
| Juncus effusus L. |
JUN.EFF |
| Lycopus europaeus L. |
LYC.EUR |
| Lythrum salicaria L. |
LYT.SAL |
| Mentha aquatica L. |
MEN.AQU |
| Mentha longifolia (L.) L. |
MEN.LON |
| Mentha spicata L. |
MEN.SPI |
| Nasturtium officinale W. T. Aiton |
NAS.OFF |
| Phalaris arundinacea L. |
PHA.ARU |
| Phragmites australis L. |
PHR.AUS |
| Polygonum hydropiper L. |
POL.HYD |
| Rorippa amphibia (L.) Besser |
ROR.AMP |
| Sagittaria latifolia Willd. |
SAG.LAT |
| Scirpus lacustris L. |
SCI.LAC |
| Sparganium erectum L. |
SPA.ERE |
| Stachys palustris L. |
STA.PAL |
| Typha angustifolia L. |
TYP.ANG |
| Typha latifolia L. |
TYP.LAT |
| Veronica beccabunga L. |
VER.BEC |
Mountain river sites (national type R03; n = 15) were located at high altitude (median: 705 m a.s.l.) and characterized by coarse substrate, rapidly running water, and shaded conditions. Macrophyte communities were dominated by helo- and hygrophytes. Mosses were recorded at only 25% of the sites.
The more variable was the dataset of semi-mountain river sites (national types R04 and R05; n = 17). It could be divided into two major subsets using Detrended Correspondence Analysis (DCA). The first subset included the aquatic moss P. riparioides; the hydrophytes Ceratophyllum submersum, Lemna minor, Myriophyllum spicatum, Potamogeton berchtoldii, and Potamogeton nodosus; and the helophytes Berula erecta, Scirpus lacustris, and Sparganium erectum. This assemblage pattern was recorded along small rivers from both northern and southern Bulgaria, at medium altitude (71–493 m a.s.l.), moderate velocity of the current, and diverse dominant substrate (sand, gravel, stones).
The second subset was represented mainly by the helophytes Echinochloa crus-galli, Juncus effusus, Lycopus europaeus, Lythrum salicaria, Mentha aquatica, Mentha longifolia, Phragmites australis, Typha latifolia, and Veronica beccabunga and the hygrophytes Cyperus longus, Epilobium hirsutum, Myosoton aquaticum, Paspalum paspaloides, and Polygonum lapathifolium. These assemblages were characteristic for small and medium sized rivers in southern Bulgaria at higher altitudes (75–837 m, median 300 m a.s.l.), coarser substrate (stones), and predominantly sunny sites.
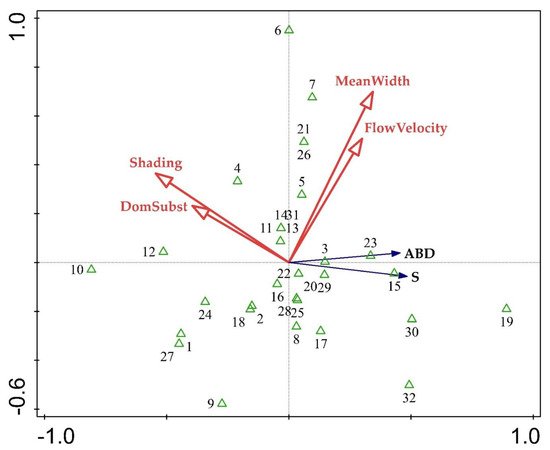
Redundancy Analysis indicated a distinct abiotic gradient (Figure 3). The gradient was related to mean river width, flow velocity, channel substrate, and shading, which represent the physical character of a river, distinguishing between more wide and rapid streams (right part of the plot) and shaded and stony streams (left part). Species richness and abundance per site increased in conditions of wider river habitats with rapidly running water, while the number of taxa and their abundance was lower in shaded sites dominated by coarser substrate.
Figure 3. RDA ordination tri-plot with species richness (S), macrophyte abundance (ABD), and four abiotic characteristics. Refer to Annex 1 for the numbering of the sites.
4. Relationship of Aquatic Macrophyte Communities and Selected Stressors
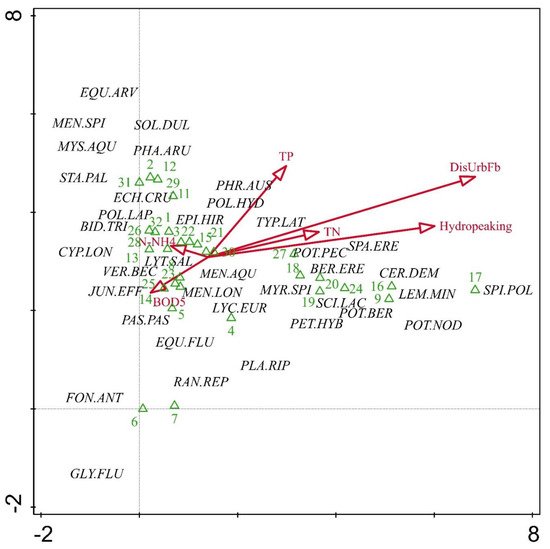
DCA was used to examine relations between macrophyte communities and stressors. The first and second DCA axes together explained 25% of the species-environmental relation (Figure 4). The first axis had the strongest positive correlation with the percent of discontinuous urban fabric presence, hydropeaking, and total nitrogen. Aquatic macrophyte assemblages in river habitats impacted by man-made structures, hydropeaking, and nitrate loading were characterized by the hydrophytes Ceratophyllum demersum, Myriophyllum spicatum, Potamogeton berchtoldii, P. nodosus, P. pectinatus, and Lemna minor. Riverbank assemblages at these sites were dominated by Berula erecta, Scirpus lacustris, and Sparganium erectum. The representative taxa of both ecomorphological types were located between the center and the edge of the plot and showed a clear relation with the first axis. The results of the DCA also suggested two homogeneous groups of sites: The larger group (close to the center of the plot) was positioned along gradients of ammonium nitrogen, total phosphorus, and organic matter. These sites featured no hydrophytes. The second group was positioned along the rest of the stressors and included mainly highly modified water bodies. Upland river sites under multiple stress lose their aquatic and rheophilous bryophyte communities. Under nutrient stress, mainly bank assemblages were supported, while sites with pronounced stress of both hydromorphological and chemical disturbance also provided conditions for the development of atypical vascular aquatic plant assemblages.
Figure 4. DCA ordination plot with selected environmental factors (DisUrbFb: discontinuous urban fabric %, TN: total nitrogen, TP: total phosphorus, N-NH4: ammonium nitrogen, BOD5: biological oxygen demand). Eigenvalue of the first axis: 0.747; second axis: 0.563. Refer to Table 1 for species codes.
Multiple regression analysis showed a strong relation of macrophyte abundance (R2 = 0.41; p < 0.05) with the six stressors. Under conditions of increasing total phosphorus, macrophyte abundance increased, while in river sites rich in total nitrogen the abundance decreased. River sites with the highest nitrogen values supported communities of helophytes and hygrophytes, while floating and submerged aquatic macrophytes were absent or recorded at low abundances. High nitrogen concentrations resulted in a decline of aquatic macrophyte abundance.