Upland rivers across Europe still exhibit undisturbed conditions and represent a treasure that we cannot afford to lose. We hypothesize that the combination of pristine and modified conditions could demonstrate biological responses along the stressor gradients. Thus, the response of aquatic macrophyte communities to anthropogenic stressors along upland rivers in Bulgaria was studied. Six stressors were selected out of 36 parameters grouped into hydromorphological, chemical variables and combined drivers (catchment land use). The stressors strongly affected species richness on the basis of biological type (bryophytes vs. vascular plants) and ecomorphological type (hydrophytes vs. helophytes). Hydrological alteration expressed by the change of the river’s base flow and altered riparian habitats has led to a suppression of bryophytes and a dominance of riverbank plant communities. Seventy-five percent of mountain sites were lacking bryophytes, and the vegetation at semi-mountainous sites was dominated by vascular plants. It can be concluded that hydropeaking, organic and inorganic pollution, and discontinuous urban structures caused important modifications in the aquatic macrophyte assemblages. Macrophyte abundance and the biological and ecomorphological type of aquatic macrophytes reflect multi-stressor effects in upland rivers.
- bryophytes
- helophytes
- macrophyte communities
- hydromorphology
- multiple stressors
1. Introduction
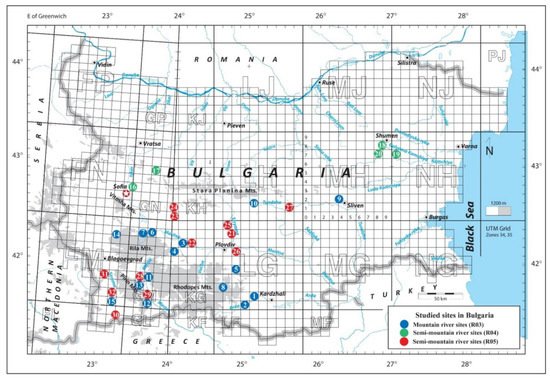
2. Studied Sites and Stressors
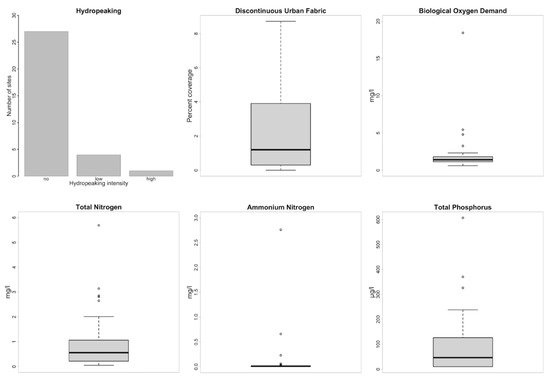
3. Aquatic Macrophyte Assemblage Patterns
| Algae | Code |
|---|---|
| Lemanea fluviatilis | LEM.FLU |
| Mosses | |
| Brachythecium rivulare Schimp. | BRA.RIV |
| Cratoneuron filicinum (Hedw.) Spruce | CRA.FIL |
| Fontinalis antipyretica Hedw. | FON.ANT |
| Leptodictyum riparium (Hedw.) Warnst. | LEP.RIP |
| Platyhypnidium riparioides (Hedw.) Dixon | PLA.RIP |
| Vascular plants | |
| Pteridophytes | |
| Equisetum arvense L. | EQU.ARV |
| Equisetum fluviatile L. | EQU.FLU |
| Equisetum sylvaticum L. | EQU.SYL |
| Equisetum telmateia Ehrh. | EQU.TEL |
| Hydrophytes | |
| Callitriche stagnalis Scop. | CAL.STA |
| Ceratophyllum demersum L. | CER.DEM |
| Ceratophyllum submersum L. | CER.SUB |
| Elodea canadensis Michx. | ELO.CAN |
| Lemna minor L. | LEM.MIN |
| Myriophyllum spicatum L. | MYR.SPI |
| Potamogeton berchtoldii Fieber | POT.BER |
| Potamogeton crispus L. | POT.CRI |
| Potamogeton nodosus Poir. | POT.NOD |
| Potamogeton pectinatus L. | POT.PEC |
| Potamogeton perfoliatus L. | POT.PER |
| Potamogeton pusillus L. | POT.PUS |
| Ranunculus trichophyllus Chaix | RAN.TRI |
| Spirodela polyrhiza (L.) Schleid. | SPI.POL |
| Hygrophytes | |
| Bidens tripartitus L. | BID.TRI |
| Cyperus longus L. | CYP.LON |
| Epilobium hirsutum L. | EPI.HIR |
| Myosoton aquaticum (L.) Moench | MYO.AQU |
| Paspalum paspalodes (Michx.) Scribn. | PAS.PAS |
| Petasites hybridus (L.) G. Gaertn. & al. | PET.HYB |
| Polygonum lapathifolium L. | POL.LAP |
| Polygonum mite Schrank | POL.MIT |
| Ranunculus repens L. | RAN.REP |
| Solanum dulcamara L. | SOL.DUL |
| Helophytes | |
| Alisma lanceolatum With. | ALI.LAN |
| Alisma plantago-aquatica L. | ALI.PLA |
| Berula erecta (Huds.) Coville | BER.ERE |
| Cyperus fuscus L. | CYP.FUS |
| Echinochloa crus-galli (L.) P. Beauv. | ECH.CRU |
| Glyceria fluitans (L.) R. Br. | GLY.FLU |
| Juncus effusus L. | JUN.EFF |
| Lycopus europaeus L. | LYC.EUR |
| Lythrum salicaria L. | LYT.SAL |
| Mentha aquatica L. | MEN.AQU |
| Mentha longifolia (L.) L. | MEN.LON |
| Mentha spicata L. | MEN.SPI |
| Nasturtium officinale W. T. Aiton | NAS.OFF |
| Phalaris arundinacea L. | PHA.ARU |
| Phragmites australis L. | PHR.AUS |
| Polygonum hydropiper L. | POL.HYD |
| Rorippa amphibia (L.) Besser | ROR.AMP |
| Sagittaria latifolia Willd. | SAG.LAT |
| Scirpus lacustris L. | SCI.LAC |
| Sparganium erectum L. | SPA.ERE |
| Stachys palustris L. | STA.PAL |
| Typha angustifolia L. | TYP.ANG |
| Typha latifolia L. | TYP.LAT |
| Veronica beccabunga L. | VER.BEC |
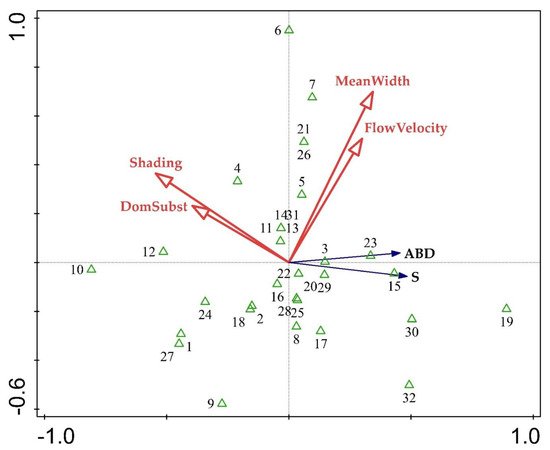
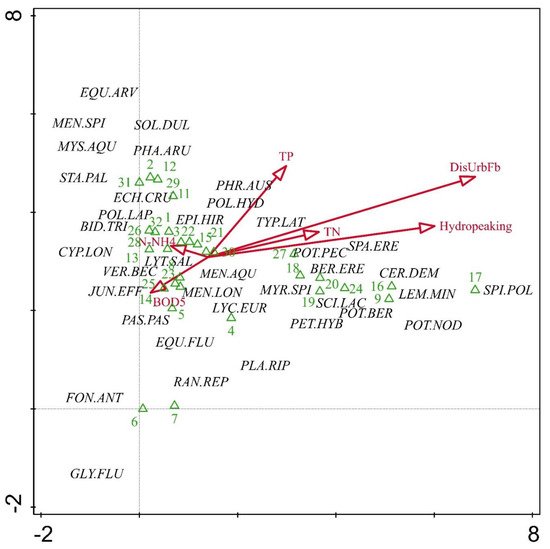
4. Relationship of Aquatic Macrophyte Communities and Selected Stressors
References
- Lemm, J.U.; Venohr, M.; Globevnik, L.; Stefanidis, K.; Panagopoulos, Y.; van Gils, J.; Posthuma, L.; Kristensen, P.; Feld, C.K.; Mahnkopf, J.; et al. Multiple stressors determine river ecological status at the European scale: Towards an integrated understanding of river status deterioration. Global Chang. Biol. 2021, 27, 1962–1975.
- Solheim, L.A.; Globevnik, L.; Austnes, K.; Kristensen, P.; Moe, J.; Persson, J.; Phillips, G.; Poikane, S.; Van De Bund, W.; Birk, S. A new broad typology for rivers and lakes in Europe: Development and application for large-scale environmental assessments. Sci. Total Environ. 2019, 697, 134043.
- European Environment Agency. European Waters—Assessment of Status and Pressures; EEA Report No 7/2018; EEA: Copenhagen, Denmark, 2018.
- Grizzetti, B.; Pistocchi, A.; Liquete, C.; Udias, A.; Bouraoui, F.; van de Bund, W. Human pressures and ecological status of European rivers. Sci. Rep. 2017, 7, 205.
- Carvalho, L.; Mackay, E.B.; Cardoso, A.C.; Baattrup-Pedersen, A.; Birk, S.; Blackstock, K.L.; Borics, G.; Borja, A.; Feld, C.K.; Ferreira, M.T.; et al. Protecting and restoring Europe’s waters: An analysis of the future development needs of the Water Framework Directive. Sci. Total Environ. 2019, 658, 1228–1238.
- Nõges, P.; Argillier, C.; Borja, Á.; Garmendia, J.M.; Hanganu, J.; Kodeš, V.; Pletterbauer, F.; Sagouis, A.; Birk, S. Quantified biotic and abiotic responses to multiple stress in freshwater, marine and ground waters. Sci. Total Environ. 2016, 540, 43–52.
- Birk, S.; Chapman, D.; Carvalho, L.; Spears, B.M.; Andersen, H.E.; Argillier, C.; Auer, S.; Baattrup-Pedersen, A.; Banin, L.; Beklioğlu, M.; et al. Impacts of multiple stressors on freshwater biota across spatial scales and ecosystems. Nat. Ecol. Evol. 2020, 4, 1060–1068.
- Springe, G.; Sandin, L.; Briede, A.; Skuja, A. Biological quality metrics: Their variability and appropriate scale for assessing streams. Hydrobiologia 2006, 566, 153–172.
- Pinto, P.; Morais, M.; Ilhéu, M.; Sandin, L. Relationships among biological elements (macrophytes, macroinvertebratesand ichthyofauna) for different core river types across Europe at two differentspatial scales. Hydrobiologia 2006, 566, 75–90.
- Hering, D.; Johnson, R.K.; Kramm, S.; Schmutz, S.; Szoskiewicz, K.; Verdonschot, P.F.M. Assessment of European streams with diatoms, macrophytes, macroinvertebrates and fish: A comparative metric-based analysis of organism response to stress. Freshw. Biol. 2006, 51, 1757–1785.
- Knight, C.G.; Staneva, M.P. The Water Resources of Bulgaria: An Overview. GeoJournal 1996, 40, 347–362. Available online: www.jstor.org/stable/41147007 (accessed on 19 July 2021).
- Cheshmedjiev, S.D.; Karagiozova, T.I.; Michailov, M.A.; Valev, V.P. Revision of River & Lake Typology in Bulgaria within Ecoregion 12 (Pontic Province) and Ecoregion 7 (Eastern Balkans) According to the Water Framework Directive. Ecol. Balk. 2010, 2, 75–96.
- Zagorchev, I. Geomophological Zonation of Bulgaria Principles and State of the Art. Comptes Rendus Acad. Bulg. Sci. 2009, 62, 981–992.
- Meshinev, T. Vegetation and Phytogeography: A Brief Characteristic. In Biogeography and Ecology of Bulgaria; Fet, V., Popov, A., Eds.; Monographiae Biologicae; Springer: Dordrecht, The Netherlands, 2007; Volume 82, pp. 581–588.
- European Union. The EU Environmental Implementation Review 2019 Country Report—Bulgaria. 2019. Available online: https://ec.europa.eu/environment/eir/pdf/report_bg_en.pdf (accessed on 7 September 2021).
