Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gilles Iacazio | + 3292 word(s) | 3292 | 2021-12-13 03:00:27 | | | |
| 2 | Peter Tang | Meta information modification | 3292 | 2021-12-22 02:40:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Iacazio, G. Terpene Mini-Path for Terpenoids Bio-Production. Encyclopedia. Available online: https://encyclopedia.pub/entry/17386 (accessed on 07 February 2026).
Iacazio G. Terpene Mini-Path for Terpenoids Bio-Production. Encyclopedia. Available at: https://encyclopedia.pub/entry/17386. Accessed February 07, 2026.
Iacazio, Gilles. "Terpene Mini-Path for Terpenoids Bio-Production" Encyclopedia, https://encyclopedia.pub/entry/17386 (accessed February 07, 2026).
Iacazio, G. (2021, December 21). Terpene Mini-Path for Terpenoids Bio-Production. In Encyclopedia. https://encyclopedia.pub/entry/17386
Iacazio, Gilles. "Terpene Mini-Path for Terpenoids Bio-Production." Encyclopedia. Web. 21 December, 2021.
Copy Citation
Terpenoids constitute the largest class of natural compounds and are extremely valuable from an economic point of view due to their extended physicochemical properties and biological activities. An alternative to produce terpenoids is the use of biotechnological tools involving, for example, the construction of enzymatic cascades (cell-free synthesis) or a microbial bio-production thanks to metabolic engineering techniques.
terpenoids bio-access
terpene mini-path
1. Introduction
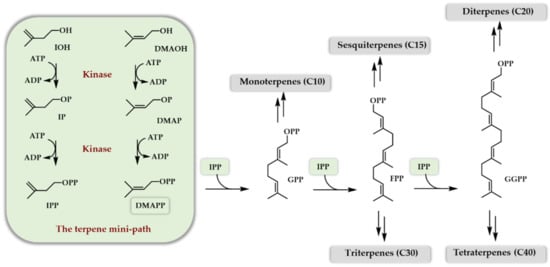
Taxol, artemisinin, menthol, camphor, cholesterol, beta-carotene, and rubber are just a few very well-known names of terpenoids of some interest for humanity. As a natural product family, terpenoids have long attracted scientific interest due to their very diversified physical and chemical properties as well as their numerous biological activities. Another point of great interest lies in their unique biosynthetic scheme. Indeed, two mechanisms, called the mevalonate pathway (MVA) and the methyl erythritol phosphate (MEP) pathway together with glycolysis, convert glucose into the two five carbon atoms, universal precursors of terpenoids: dimethylallyl diphosphate (DMAPP) and isopentenyl diphosphate (IPP). Both pathways involve an 18 enzymes cascade. The MVA pathway is mainly found in eukaryotes and archaebacteria, whereas the MEP pathway is mainly found in eubacteria and chloroplasts. Once synthesized, DMAPP and IPP are condensed together to generate, in an extension process, linear diphosphates of different lengths characterized by a carbon number multiple of five. The extraordinary structural diversity of terpenoids comes next. Indeed, these linear diphosphates are first cyclized by numerous terpene synthases/cyclases, generating cyclic hydrocarbons but also alcohols or ethers (see refs [1][2][3][4][5][6] for a detailed view of their mechanism of action), giving rise to monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), sesterterpenes (C25), triterpenes (C30), tetraterpenes (C40), and higher homologs depending on the linear diphosphate used as a starter unit (DMAPP, geranyl diphosphate GPP, farnesyl diphosphate FPP, geranylgeranyl diphosphate GGPP and the number of extension units added (IPP). A second layer of diversity is then added when the initially formed scaffold is enzymatically decorated with a myriad of oxidases (cytochrome P450, α-ketoglutarate dependent dioxygenases), glyco- and acyl-transferases, as well as dehydrogenases and halogenases. These two steps of modification, cyclisation and decoration, are, thus, the basis of terpenoids’ structural diversity. The first step usually involves a single enzyme, while the second, depending on the final product structure, may involve more than ten enzymes. As a projecting example, we provide here a simplified biosynthesis scheme of taxol (Scheme 1), the whole sequence, particularly during the decoration phase, being actually not entirely known [7].
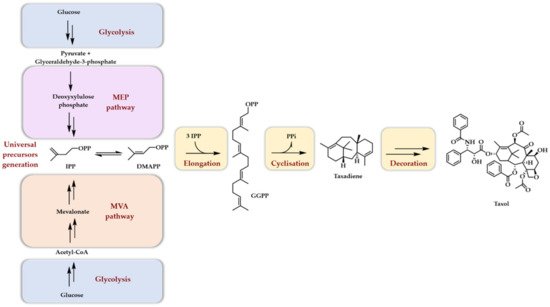
Scheme 1. Simplified biosynthetic scheme of terpene biosynthesis with a special focus put on taxol biosynthesis. It should be noted that, in plants, diterpenoids (such as taxol) arise mainly from the plastidial MEP pathway.
It should also be noted that DMAPP, as well as diphosphates of higher length, such as GPP, FPP, and GGPP, can be coupled to natural molecules of different biosynthetic origins, such as amino acids or polyphenols, and further processed, offering another source of structural diversity.
If a one- or two-step enzymatic phosphorylation access to DMAPP and IPP becomes available starting from DMAOH and IOH, then the paradigm of terpenoids’ bio-production could be changed and give rise to a new and easy way to the industrial access of those outstanding chemicals. Indeed, if successful, the implementation of this new artificial pathway offers the possibility to decouple microbial growth (use of glucose or glycerol as carbon and energy sources) and terpenoid production (use of DMAOH and IOH as biosynthetic carbon), hence simplifying the process of optimization. In addition, the use of only one or two enzyme(s) to doubly phosphorylate DMAOH and IOH makes this artificial route usable both in vivo and in vitro due to its extreme simplicity. Best of all, DMAOH and IOH are both readily available bulk chemicals and can potentially be bio-sourced. The idea became true when four groups reported independently in 2019 [8][9][10][11] the capacity of a two enzymes cascade to generate DMAPP and IPP from DMAOH and IOH in order to produce, either in vitro or in vivo, various terpenoids. (Scheme 2).
Scheme 2. Simplified enzymatic access to DMAPP and IPP, as well as terpenoids, through the TMP.
2. Premises
The TMP is a priori totally artificial. Indeed, DMAOH and IOH are not very common chemicals found in living organisms and, if generated, they are considered to occur from DMAPP and IPP by (di)phosphatase action rather than the opposite. Nevertheless, some experimental evidence has been provided that DMAOH and IOH can be used either in a mixture or individually by some microorganisms to obtain higher titers in terpenoids when exogenously added to the culture medium. It should be noted that no enzymes were described nor suggested to be responsible for the incorporation of the two alcohols in these initial studies.
3. One Enzyme Access to DMAPP/IPP
3.1. The Isopentenyl Phosphate Kinase Enzyme
Before being recognized as part of a modified mevalonate pathway in archaea [12], isopentenyl phosphate kinase (IPK) was first detected in E. coli and in Mentha piperita [13] and was believed at the time to be the last enzymatic step of the not-yet-elucidated MEP pathway [13]. Using the recombinant and partially purified E. coli IPK (IPKEc), it was demonstrated that the best tested enzyme substrate in the presence of ATP was isopentenyl phosphate (IP) generating isopentenyl diphosphate (Scheme 3).
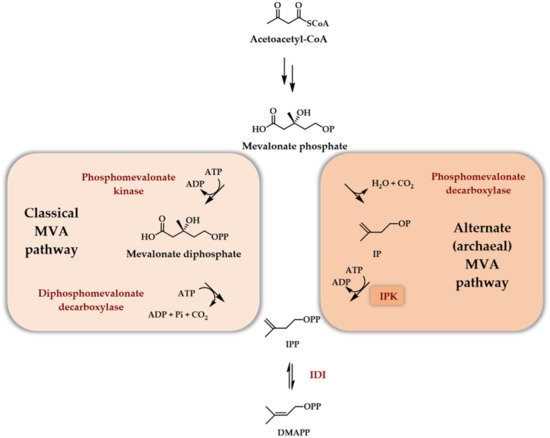
Scheme 3. Role of IPK in the alternate archaeal MVA pathway.
Later, it was recognized by bioinformatic studies that each sequenced plant genome possessed an IPK encoding gene [14]. It is, therefore, relevant to consider what the physiological role of this enzyme is in plants. Recently, some studies have demonstrated a potential physiological role of plant IPKs in conjunction with hydrolases from the Nudx family, both enzymes being supposed to be involved in the regulation of DMAPP/DMAP and IPP/IP ratios in plant cytosol ([15], reviewed in [14]). Indeed, the ability of DMAP and IP to competitively inhibit farnesyl diphosphate synthase and the demonstrated presence of both IPK and phosphatase acting specifically on DMAPP and IPP but not on DMAP or IP led to a regulatory scheme [15]. This latter describes the central role of the DMAP/IP pair in the regulation of the biosynthesis of terpenoids through the cytosolic MVA pathway, at least in plants (Scheme 4).
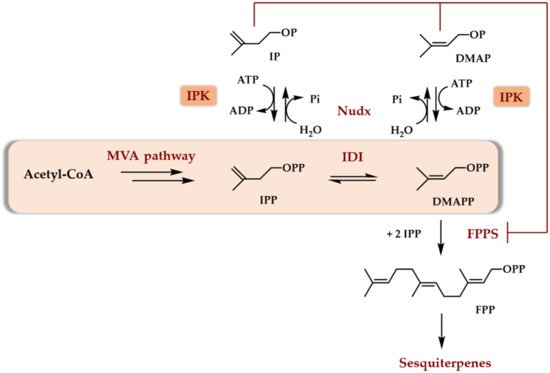
Scheme 4. Potential physiological role of IPKs (and of Nudx) in the regulation of sesquiterpenoids biosynthesis in plants. IDI stands for isopentenyl diphosphate isomerase. Nudx stands for Nudx phosphatase. FPPS stands for farnesyl diphosphate synthase (adapted from [15]).
3.2. One Enzyme Double Phosphorylation of DMAOH and IOH
The one enzyme double phosphorylation of DMAOH and IOH is obviously the shortest route to access the universal precursors DMAPP and IPP. Two papers have been devoted to such a restricted cascade, both taking advantage of the ability of IPKs to catalyze this double phosphorylation. In one case, the report is based on protein engineering of IPKs by analysis of amino acid coevolution [16]; the other is focused on screening of IPK biodiversity [17]. Liu et al. were the first to use the ability of IPKs to catalyze, in addition to their normal IP kinase activity leading to IPP, another kinase activity on DMAOH, potentially leading to the construction of a one enzyme two step access to DMAPP [16]. It should be noted that the DMAOH kinase catalytic efficiency of Thermoplasma acidophilum IPK (IPKTa) was three orders of magnitude lower than the normal IP kinase activity [16][18]. In order to improve the DMAOH kinase activity, the coevolution of the IPK protein sequences was analyzed and potential amino acid positions were detected that can, if mutated, increase the catalytic activity of IPKTa towards DMAOH. Single, double, or triple mutants were generated, the best ones showing up to an eight-fold increase in catalytic activity. This protein engineering approach was used to produce in vivo (E. coli) β-carotene. A maximum of 3.78 mg of β-carotene per gram of dry cell weight was obtained in the best case, involving a triple mutant of IPKTa.
As part of a larger project, various IPKs extracted from biodiversity were tested as potential catalysts to be produced in E. coli in a soluble active form, usable at pH 7, 37 °C, and with ATP as a phosphorylating agent. From the 93 over-expressed proteins, 66 were correctly produced after IPTG addition, but only 24 were soluble. Five IPKs exhibiting high isopentenyl phosphate kinase activity with low sequence similarity to IPKTa were further chosen and their genes combined individually with the genes encoding the enzymes involved in the generation of lycopene (crtE, crtB, and crtI). When adding DMAOH and IOH to the culture medium, a maximum 11-fold increase in carotenoid content was obtained with the IPK of Methanococcus maripaludis as a catalyst. Unexpectedly, it was, furthermore, observed that the main formed carotenoid was neurosporene (up to 94%) at a concentration of 0.7 mg per gram of dry cell weight [17]. In both cases [16][17], the production of carotenoids has been proved to be associated with the expression of IPKs.
These two reports are, to our knowledge, the only ones dealing with a one enzyme TMP to generate in vivo both DMAPP and IPP from DMAOH and IOH. Unfortunately, regarding whether IPKs are efficient enzymes to transform DMAP and IP into DMAPP and IPP, their catalytic efficiency dropped drastically when tested as DMAOH/IOH kinase. As an IPK having equivalent and high activities for both phosphorylation steps is yet to be found or created, various teams used promiscuous enzymes in order to catalyze the first DMAOH/IOH phosphorylation step (no known enzymes being dedicated to this transformation in nature), in combination with an IPK, in order to generate an efficient two enzyme TMP.
4. Two Enzyme Access to DMAPP/IPP
4.1. What Enzyme to Be Coupled with an IPK
As mentioned earlier, to develop an efficient and short artificial TMP, it is necessary to couple a DMAP/IP kinase to an IPK in order to generate the two C5 corresponding diphosphates. One option, adopted in two of the first reports on the TMP, was to use a phosphatase to perform the first phosphorylation step [9][10]. Indeed, phosphatases catalyze the generally reversible hydrolysis of organic phosphates, leading to an alcohol and a phosphate ion. It was demonstrated that bacterial acidic phosphatases of the PhoN family were able to catalyze the reverse reaction, i.e., the synthesis of monophosphates starting from alcohols, and especially primary ones, using ATP or diphosphate as a phosphorylating agent [19]. Of particular relevance was the fact that such an enzyme was found to be able to catalyze the formation of isopentanyl phosphate from isopentanol, the hydrogenated equivalent of DMAOH and IOH [19].
4.2. The TMP In Vitro—Purified Enzymes
The first report dealing with the use of purified enzymes to access terpenoids from C5 alcohols demonstrated that the combination of a phosphatase and an IPK could generate DMAPP starting from DMAOH [10]. As a proof of concept, the former was used as a prenyl donor to generate a cytotoxic prenylated diketopiperazine compound, tryprostatin B, using chemically synthesized brevianamide F (BF) and prenyl transferase FtmPT1 from Aspergillus fumigatus as a catalyst (Scheme 5).
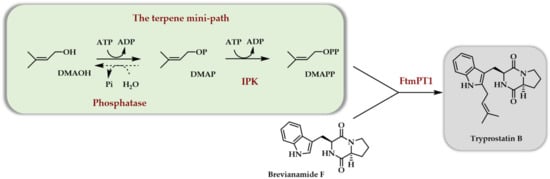
Scheme 5. First in vitro use of the TMP to access tryprostatin B in a three enzymes-one pot cascade [10].
A large optimization of the three enzymes cascade allowed to totally transform BF into tryprostatin B in 24 h on a 10 mM scale. The main influencing parameters were found to be the presence of an ATP recycling system, an increase in DMAOH concentration versus BF concentration, a double ATP addition during the reaction, a decreased concentration in phosphatase, and a higher concentration in prenyl transferase FtmPT1 as compared to initial conditions. This optimization was made necessary by the use of a phosphatase for the first enzymatic step. Indeed, it was demonstrated that this phosphatase was able to quickly hydrolyze any of the phosphates or diphosphates added in the reaction medium or produced during the reaction (DMAP, DMAPP, PEP, AMP, ADP, ATP). As a general rule, influencing parameters acting either to slow down phosphate and diphosphate hydrolysis or to maintain a high level in phosphorylating agent (low phosphatase activity, ATP regeneration system, addition of ATP) or to promote the production and use of DMAPP (higher DMAOH concentration, higher prenyl transferase activity) had to counteract phosphatase activity. While the proof of concept of the in vitro usefulness of the TMP was completed and a final concentration of tryprostatin B of ~3 g/L was obtained, the TMP using a phosphatase as the first enzyme did not appear to be the best option. It should be noted that the used enzymes were freshly purified and not frozen.
Soon after [20], a paper described the use of the Arabidopsis thaliana IPK in combination with a true kinase, the choline kinase from S. cerevisiae, as the first enzyme of the TMP, called here the IUP. The combination of these two kinases with IDI (isopentenyl diphosphate isomerase), FPPS from E. coli, and various terpene synthases (limonene synthase, amorphadiene synthase, and valencene synthase) allowed the in vitro production of the corresponding hydrocarbons (Scheme 6).
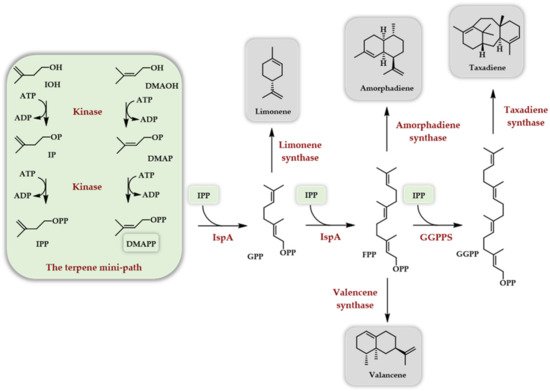
Scheme 6. In vitro access to various terpenes thanks to the use of the TMP constitute of the choline kinase from S. cerevisiae and the Arabidopsis thaliana IPK [20].
Taxadiene synthesis was also attempted using the GGPPS (geranylgeranyl diphosphate synthase) from Taxus canadensis and the taxadiene synthase from Taxus brevifolia. After the optimization of the various enzyme concentrations, as well as of ATP, DTT, and Mg2+ concentrations and other parameters, such as using only IOH with IDI versus a mix of DMAOH and IOH without IDI, a final yield of 220 mg/L in taxadiene was achieved in 9 h with a 65% conversion of added IOH.
In 2020, two papers appeared, both taking advantage of the use of another true kinase (the ThiM kinase from E. coli, see above) to phosphorylate DMAOH and IOH into DMAPP and IPP in conjunction with the Methanocaldococcus jannaschii IPK [21][22]. In the former case, the formed DMAPP and IPP were used as substrates of different linear prenyl transferases (Scheme 6) to access (E,E)-FPP, (Z,Z)-FPP, GPP, and GGPP. Implementing a terpene synthase/cyclase further afforded, depending on the used enzyme, (S)-germacrene D, (-)-germacradiene-4-ol, amorpha-4,11-diene, and 7-epi-zingiberene (Scheme 7). The TMP has also been exploited to generate, from homologs of DMAOH and IOH, various linear diphosphates homologous to FPP. Some of them were then used as substrates for germacrene D and amorphadiene sesquiterpene synthases, providing the corresponding unnatural terpenoids [22].
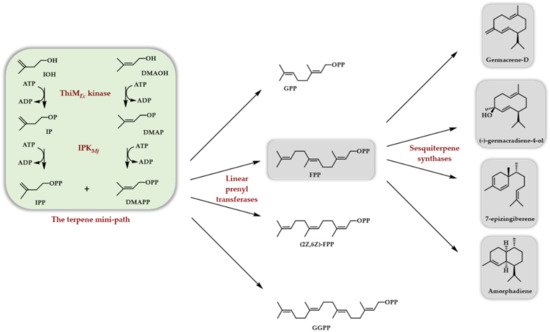
Scheme 7. In vitro access to various sesquiterpenes thanks to the use of the TMP constituted of the ThiM kinase from E. coli and the Methanocaldococcus jannaschii IPK.
The second article [21] deals with the generation of cannabinoids, implying many more enzymatic steps than previous reports (12 enzymes in total). This demonstrated that the in vitro enzymatic production of natural products of mixed biosynthetic origin (polyketide/terpene in that case) could be envisioned seriously thanks to the reduced number of enzymes of the TMP. As far as the terpene part is concerned, GPP was produced with a four enzymes cascade starting from IOH using ThiMEc, IPKMj, IDIEc, and FPPSGs S82F. The latter, a point mutant of FPPS from Geobacillus stearothermophilus, no more catalyzed the formation of FPP but rather of GPP. This so-called ISO module was combined with an ATP regeneration module employing acetyl-phosphate as a sacrificial phosphate donor. The aromatic polyketide module affords either the olivetolic or divarinic acids depending on the length of the hydrocarbon side chain and, when combined with GPP through the cannabinoid module, they afford cannabigerolic acid at 480 mg/L or cannabigerovarinic acid at 580 mg/L titers on a 1 mL scale in 10 h (Scheme 8).
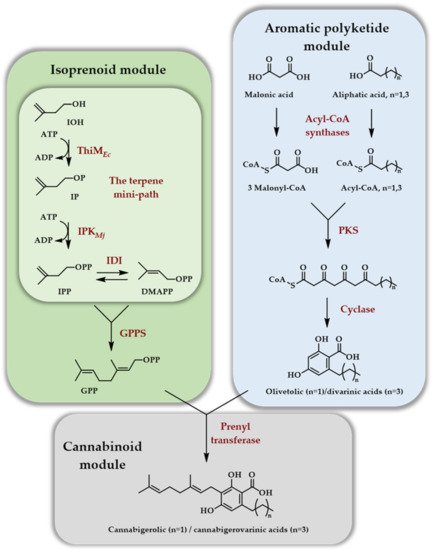
Scheme 8. In vitro access to cannabinoids thanks to the TMP involving the ThiM kinase from E. coli and the Methanocaldococcus jannaschii IPK, as well as an aromatic polyketide module and a cannabinoid module. The ATP regeneration module, as well as ATP consumption for the various modules, except for kinases, are omitted (adapted from [21]).
These four examples prove that the TMP offers, compared to the MVA and MEP pathways, the possibility to easily synthesize terpenes and terpenoids in vitro using purified enzymes. Although the enzyme purification is a time-consuming and costly process, the drastic reduction in the number of enzymes needed to access DMAPP and IPP thanks to the TMP (two instead of eighteen) can be exploited to access various terpenoids and could be of interest in the discovery of new terpene synthases as well as in the determination of their substrate promiscuity.
4.3. The TMP In Vivo
4.3.1. Escherichia coli as Host
The very first report on a two-step access to DMAPP/IPP (IUP) was mainly dedicated to the in vivo synthesis of lycopene and taxadiene (valencene, limonene, miltiradiene, and amorphadiene synthesis were also attempted) in E. coli by the use of a choline kinase from Saccharomyces cerevisiae and the Arabidopsis thaliana IPK as the two kinases of the artificial pathway [8]. The two corresponding genes were incorporated in an operon in combination with an idi gene to balance the DMAPP/IPP ratio. Two plasmids were thus generated, one with a constitutive promoter, the second with an inducible promoter. When combined with a plasmid bearing a lycopene operon, the former outperformed, the best conditions being the use of 25 mM IOH. A further optimization of the plasmid copy number, as well as the promoter strength of the lycopene operon, led to lycopene content of 8000 ppm in a short time (12 h) following induction (Scheme 9A). When taxadiene production was attempted, a 15 mg/L concentration was obtained after 48 h of culture (Scheme 9A). A very interesting result has come from this pioneer work. By analyzing the intracellular concentration of various phosphates and diphosphates (IP, IPP, GPP, FPP, GGPP), it was demonstrated that the flux to IPP using the so-called IUP pathway is larger than that of the native MEP pathway. This also led to the intracellular accumulation of IPP and GGPP, the flux of the IUP being larger than the ones of the downstream lycopene and taxadiene operons.
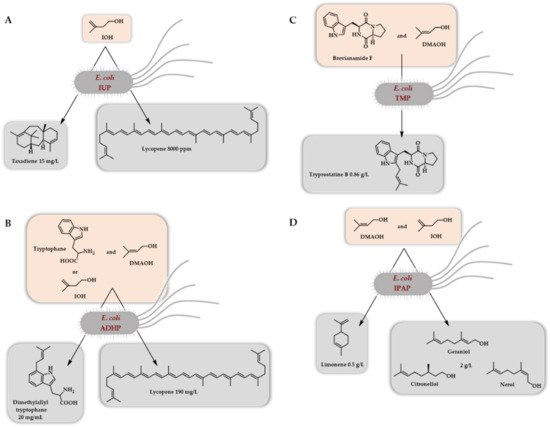
Scheme 9. Initial demonstration of the in vivo access to terpenoids (E. coli) thanks to the so-called isopentenol utilization pathway (A), the alcohol-dependent hemiterpene pathway (B), the terpene mini-path (C), or the isoprenoid alcohol pathway (D) using a two enzymes cascade and starting from DMAOH/IOH to access DMAPP/IPP.
4.3.2. Yeasts as Host
Very recently, some reports using the TMP together with an engineered MVA pathway described the production of lycopene, geranylgeraniol, and α-farnesene in various yeasts (Scheme 10). Luo et al. [23] took advantage of the oleaginous nature of the yeast Yarrowia lipolytica to produce lycopene with the rationale that the lipid body of this yeast species could store more hydrophobic lycopene than the membranes. It was demonstrated that adding the genes coding for choline kinase and IPK results in enhanced intracellular production of IPP/DMAPP upon IOH addition. When the genes coding for lycopene biosynthesis (crtE, crtB, and crtI from Lamprocystis purpurea) as well as the gene coding IDI from Pseudoescherichia vulgaris were introduced in Y. lipolytica strains, enhanced terpenoid precursors content (GPP, FPP, GGPP), as well as higher lycopene titers, were observed for the strain harboring the TMP upon IOH addition. Enhancement of lycopene titer was further observed when lipid synthesis was promoted either by overexpression of ACC1 and DGA1 (coding, respectively, for an acetyl-CoA carboxylase and a diacylglycerol acyltransferase) or by palmitic acid addition to the culture medium (lycopene titers of ~900 and ~600 mg/L, respectively, were obtained). In order to improve the utilization of intracellular FPP and GGPP, the introduction of an additional copy of an idi gene and the overexpression of erg20, encoding an endogenous FPPS, combined with the addition of palmitic acid in the culture medium, made it possible to reach a lycopene titer of 1.6 g/L in shaken flasks. A final optimization of the palmitic acid concentration (30 mM) and the finding that IOH addition is optimal two days after inoculation led to a final lycopene titer of 4.2 g/L in batch reactor after an 11-day cultivation.
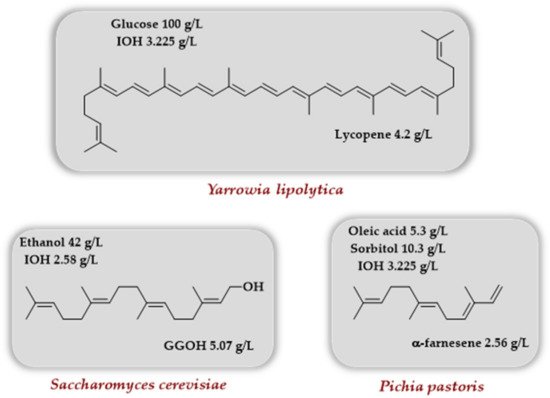
Scheme 10. Use of various yeasts to access lycopene, geranylgeraniol, and α-farnesene involving the TMP in conjunction with native or engineered MVA pathway.
References
- Cane, D.E. Enzymatic Formation of Sesquiterpenes. Chem. Rev. 1990, 90, 1089–1103.
- Christianson, D.W. Structural Biology and Chemistry of the Terpenoid Cyclases. Chem. Rev. 2006, 106, 3412–3442.
- Dickschat, J.S. Isoprenoids in Three-Dimensional Space: The Stereochemistry of Terpene Biosynthesis. Nat. Prod. Rep. 2011, 28, 1917–1936.
- Christianson, D.W. Structural and Chemical Biology of Terpenoid Cyclases. Chem. Rev. 2017, 117, 11570–11648.
- Tantillo, D.J. Biosynthesis via Carbocations: Theoretical Studies on Terpene Formation. Nat. Prod. Rep. 2011, 28, 1035–1053.
- Tantillo, D.J. Importance of Inherent Substrate Reactivity in Enzyme-Promoted Carbocation Cyclization/Rearrangements. Angew. Chem. Int. Ed. 2017, 56, 10040–10045.
- Xiong, X.; Gou, J.; Liao, Q.; Li, Y.; Zhou, Q.; Bi, G.; Li, C.; Du, R.; Wang, X.; Sun, T.; et al. The Taxus Genome Provides Insights into Paclitaxel Biosynthesis. Nat. Plants 2021, 7, 1026–1036.
- Chatzivasileiou, A.O.; Ward, V.; McBride, E.S.; Stephanopoulos, G. Two-Step Pathway for Isoprenoid Synthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 506–511.
- Lund, S.; Hall, R.; Williams, G.J. An Artificial Pathway for Isoprenoid Biosynthesis Decoupled from Native Hemiterpene Metabolism. ACS Synth. Biol. 2019, 8, 232–238.
- Couillaud, J.; Rico, J.; Rubini, A.; Hamrouni, T.; Courvoisier-Dezord, E.; Petit, J.-L.; Mariage, A.; Darii, E.; Duquesne, K.; de Berardinis, V.; et al. Simplified in Vitro and in Vivo Bioaccess to Prenylated Compounds. ACS Omega 2019, 4, 7838–7849.
- Clomburg, J.M.; Qian, S.; Tan, Z.; Cheong, S.; Gonzalez, R. The Isoprenoid Alcohol Pathway, a Synthetic Route for Isoprenoid Biosynthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 12810–12815.
- Dellas, N.; Thomas, S.T.; Manning, G.; Noel, J.P. Discovery of a Metabolic Alternative to the Classical Mevalonate Pathway. eLife 2013, 2, e00672.
- Lange, B.M.; Croteau, R. Isopentenyl Diphosphate Biosynthesis via a Mevalonate-Independent Pathway: Isopentenylmonophosphate Kinase Catalyzes the Terminal Enzymatic Step. Proc. Natl. Acad. Sci. USA 1999, 96, 13714–13719.
- Zhou, F.; Pichersky, E. More is Better: The Diversity of Terpene Metabolism in Plants. Curr. Opin. Plant Biol. 2020, 55, 1–10.
- Henry, L.K.; Gutensohn, M.; Thomas, S.T.; Noel, J.P.; Dudareva, N. Orthologs of the Archaeal Isopentenyl Phosphate Kinase Regulate Terpenoid Production in Plants. Proc. Natl. Acad. Sci. USA 2015, 112, 10050–10055.
- Liu, Y.; Yan, Z.; Lu, X.; Xiao, D.; Jiang, H. Improving the Catalytic Activity of Isopentenyl Phosphate Kinase through Protein Coevolution Analysis. Sci. Rep. 2016, 6, 24117.
- Rico, J.; Duquesne, K.; Petit, J.-L.; Mariage, A.; Darii, E.; Peruch, F.; de Berardinis, V.; Iacazio, G. Exploring Natural Biodiversity to Expand Access to Microbial Terpene Synthesis. Microbial. Cell Fact. 2019, 18, 23.
- Grochowski, L.L.; Xu, H.; White, R.H. Methanocaldococcus jannaschii Uses a Modified Mevalonate Pathway for Biosynthesis of Isopentenyl Diphosphate. J. Bacteriol. 2006, 188, 3192–3198.
- Van Herk, T.; Hartog, A.F.; van der Burg, A.M.; Wever, R. Regioselective Phosphorylation of Carbohydrates and Various Alcohols by Bacterial Acid Phosphatases; Probing the Substrate Specificity of the Enzyme from Shigella flexneri. Adv. Synth. Catal. 2005, 347, 1155–1162.
- Ward, V.C.A.; Chatzivasileiou, A.O.; Stephanopoulos, G. Cell Free Biosynthesis of Isoprenoids from Isopentenol. Biotechnol. Bioeng. 2019, 116, 3269–3281.
- Valliere, M.A.; Korman, T.P.; Arbing, M.A.; Bowie, J.U. A Bio-Inspired Cell-Free System for Cannabinoid Production from Inexpensive Inputs. Nat. Chem. Biol. 2020, 16, 1427–1433.
- Johnson, L.A.; Dunbabin, A.; Benton, J.C.R.; Mart, R.J.; Allemann, R.K. Modular Chemoenzymatic Synthesis of Terpenes and their Analogues. Angew. Chem. Int. Ed. 2020, 59, 8486–8490.
- Luo, Z.; Liu, N.; Lazar, Z.; Chatzivasileiou, A.; Ward, V.; Chen, J.; Zhou, J.; Stephanopoulos, G. Enhancing Isoprenoid Synthesis in Yarrowia lipolytica by Expressing the Isopentenol Utilization Pathway and Modulating Intracellular Hydrophobicity. Metab. Eng. 2020, 61, 344–351.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Entry Collection:
Organic Synthesis
Revisions:
2 times
(View History)
Update Date:
22 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




