Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Peter Crisologo | + 1589 word(s) | 1589 | 2021-11-03 10:33:56 | | | |
| 2 | Jason Zhu | Meta information modification | 1589 | 2021-12-22 02:17:41 | | | | |
| 3 | Katie Lynn Rubitschung | Meta information modification | 1589 | 2021-12-28 02:28:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Crisologo, P.; Rubitschung, K. Pathophysiology and Clinical Assessment of DFI. Encyclopedia. Available online: https://encyclopedia.pub/entry/17363 (accessed on 15 January 2026).
Crisologo P, Rubitschung K. Pathophysiology and Clinical Assessment of DFI. Encyclopedia. Available at: https://encyclopedia.pub/entry/17363. Accessed January 15, 2026.
Crisologo, Peter, Katie Rubitschung. "Pathophysiology and Clinical Assessment of DFI" Encyclopedia, https://encyclopedia.pub/entry/17363 (accessed January 15, 2026).
Crisologo, P., & Rubitschung, K. (2021, December 21). Pathophysiology and Clinical Assessment of DFI. In Encyclopedia. https://encyclopedia.pub/entry/17363
Crisologo, Peter and Katie Rubitschung. "Pathophysiology and Clinical Assessment of DFI." Encyclopedia. Web. 21 December, 2021.
Copy Citation
In the past 30 years, diabetic foot infections (DFI) have become increasingly prevalent due to the rising incidence of diabetes mellitus (DM). Twenty percent of diabetes-related hospital admissions in the U.S. are from DFI, which is typically introduced by direct inoculation through a traumatic entry site in an insensate foot.
diabetic foot infection
Pathophysiology
Clinical Assessment
1. Diabetic Susceptibility to Diabetic Foot Infections (DFI)
Several pathological factors place diabetic patients at increased risk for foot infections, including diabetic neuropathy, vascular insufficiency, and immunological dysfunction [1]. A strong link has been reported between diabetic neuropathy and foot ulceration [2]. Diabetic neuropathy is a complex polyneuropathy consisting of peripheral, motor, and autonomic neuronal damage [3].
Peripheral neuropathy is prevalent in 10.9–32.7% of diabetic patients in the U.S. [4][5]. Minor foot trauma from sources such as ill-fitting shoes or injury goes unnoticed from lack of sensation [6]. When repeated or left unattended, this trauma may lead to ulceration (Figure 1) and subsequent infection. Muscle atrophy, foot deformity (Charcot arthropathy, claw foot, pes cavus, hallux valgus), and gait abnormalities are caused by motor neuropathy resulting from the loss of myelinated fibers [7]. These biomechanical changes predispose neuropathic patients to foot ulceration due to increased pressure and shearing. Additionally, a study by Lung et al. suggests that moderate-to-fast walking intensity decreases plantar stiffness and increases risk of foot ulceration compared to walking at slower speeds [8]. Charcot arthropathy develops in 13% of patients with neuropathy. It can result in fractures, dislocations, and fracture dislocations causing profound deformity and a rocker-bottom appearance of the plantar foot, due to bone breakdown and joint collapse [9][10]. Autonomic neuropathy in the lower limb causes vasodilation and excess warmth in the foot. Moreover, impaired neuronal control of the sweat glands reduces perspiration (anhidrosis), leading to dry skin that is prone to fissure or callus and increasing the risk of developing a foot wound [11][12]. Diabetic patients may also experience vascular insufficiency (micro and macrovascular) and immunological dysfunction, which further predisposes them to ulceration, impaired healing, and infection (Figure 1).
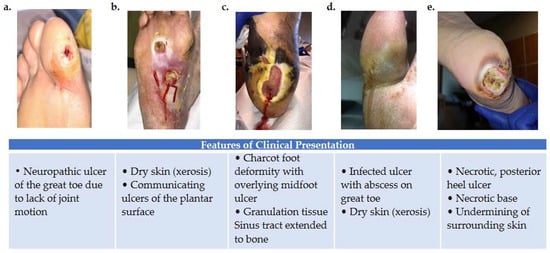
Figure 1. Variations in foot wound location, presentation, and severity as illustrated by images (a–e). (a) Patient presenting with neuropathic ulcer under the proximal interphalangeal joint of the hallux. The ulcer is related to lack of joint motion at the first metatarsophalangeal joint. (b) Patient presenting with an infected ulcer following the flexor tendons of the foot. Notice a blow lesion at the plantar arch. Dry skin (xerosis) is a sign of autonomic neuropathy. (c) Patient presenting with Charcot foot deformity and overlying midfoot ulcer. Macerated skin around the edges of the ulcer and a sinus tract that extends to the bone is also seen. (d) Patient with an infected ulcer with abscess on the great toe and xerosis suggesting autonomic neuropathy. (e) Patient with a posterior heel ulcer containing a necrotic base and undermining of surrounding skin.
2. Clinical Diagnostic Tests
Several diagnostic tests are available to help with the clinical assessment of diabetic foot infections (DFI), including the probe-to-bone test, biopsy, and assessment of inflammatory markers (ESR and CRP). The probe-to-bone test is routinely used during physical examination of the patient to evaluate the potential for OM [13]. In this procedure, a sterile tip probe is introduced through the ulcer to determine if bone is palpable. A solid/gritty end-point is considered positive for OM [14]. Suspected infections may be evaluated for microorganisms by sampling the wound site with an ulcer swab, soft tissue biopsy, or bone biopsy.
Many have argued that the results of these tests should not be independently used for diagnosis and should be only used as a screening method [15]. A negative test result in any of these cases does not exclude the diagnosis of DFI and a positive result may be misleading. For instance, soft tissue biopsies or swab cultures only assess the superficial flora and may not reflect the full extent of infection [16]. Bone biopsies are also limited because the technique requires trained personnel and it is prone to sampling errors. Moreover, there is no standardized definition of a positive bone biopsy and many classifications exist, each with its own merits and weaknesses—further convoluting the problem [16][17]. Historically, classification systems did not include the presence of OM as an indication of infection severity, and it is well known that the presence of OM negatively impacts the outcomes of diabetic foot infections [18]. Without a universal classification system, determinants of infection severity may vary, leading to a diagnostic disagreement among physicians. The accuracy of tests such as the probe-to-bone test relies on the prevalence of OM in the patient cohort being examined. For example, a group of hospitalized patients with severe infection may have a high positive predicative value using these methods, while a group of outpatients with low likelihood of OM may have a low positive predicative value [19]. These factors, as well as others, have encouraged advancements in understanding of the molecular mechanisms in DFI.
3. Molecular Mechanisms of DFI Features
All of the currently available diagnostic tests rely on the assessment of changes in DFI at a tissue level (Figure 2); however, such changes are only detectable when damage has already been inflicted. A strong understanding of the molecular changes that precede visual tissue damage is required to advance the diagnosis and treatment of DFI.
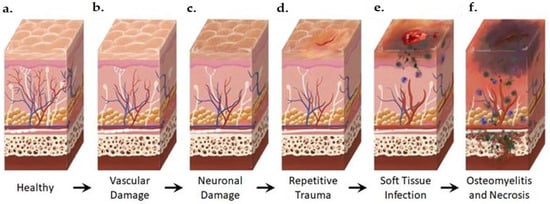
Figure 2. Anatomical Changes Associated with Diabetic Foot Pathophysiology. Diabetic neuropathy is a multi-faceted polyneuropathy related to an increased risk of ulceration, infection, and amputation. Sustained hyperglycemia damages the endothelial lining of the blood vessels in previously healthy tissue (a), leading to impaired circulation (b). Without sufficient vascular support, nerves die off and the skin may become dry and cracked as sweat secretions decrease (c). In the event of injury, numbness in the foot due to neuronal ischemia may mean that insults go undetected for some time (d). Fissures in the dried skin can harbor microorganisms, increasing the likelihood of wound infection. Initial microbial invasion of the trauma site leads to inflammation, vasodilation, and soft tissue necrosis (e). Decreased vascularization compromises immune response to infection and prolongs healing time. If the infection persists, usually because of delayed care or ineffective treatment, microbes may invade bone tissue, leading to osteomyelitis and bone deformation (f). White: neurons, red: arteries, blue: veins, purple: polymorphonuclear lymphocytes, green: microorganisms.
Bone deformities in the foot such as Charcot arthropathy occur when bone destruction from osteoclastic resorption exceeds bone building from osteoblastic recruitment. This breakdown, radiographically visualized as osteolysis, is mediated through the inflammatory RANKL (receptor activator of the nuclear factor-kappa B ligand) pathway and has been identified as an important pathway in the pathogenesis of Charcot foot. When RANKL concentration is higher than its competitive antagonist, OPG (osteoprotegerin), osteoclastogenesis is stimulated, affecting increased osteoclast activity, osteolysis, and deformity [20][21][22].
Arterial atherosclerosis, excessive leukocyte adhesion, increased vascular permeability, impaired hemostasis, and altered proliferation and apoptosis of vascular cells are all factors contributing to diabetic vascular insufficiency [23]. Poorly controlled glucose levels activate the polyol glucose metabolism pathway in which glucose is reduced to sorbitol and subsequently oxidized to fructose. Excess fructose and sorbitol lead to increased osmotic molality and ultimately osmotic stress [24]. Competitive consumption of NADPH and decreased nitric oxide synthetase activity also contribute to this challenge, as low nitric oxide concentration stimulates vessel constriction [25].
In addition to diminished perfusion, immune dysfunction has also been reported [26]. Diabetic patients frequently present with defective neutrophil function irrespective of glycemic status, including disrupted migration patterns associated with reduced production of chemotactic factors, amplified generation of reactive oxygen species, and abated phagocytosis from complement system dysfunction. Collectively, these changes facilitate pathogen invasion and infection development [27]. One retrospective clinical study demonstrated that the ratio of lymphocytes to neutrophils, platelets, and monocytes could predict the need for amputation due to DFI [28].
The innate immune system is a major source of cytokines. Macrophages and dendritic cells may produce cytokines in response to damage-associated molecular patterns (DAMPS) through the activation of pattern recognition receptors (PRRs). These inflammatory cytokines (such as TNFα, IL-1, IL-6, and interferon-ϒ) trigger osteoblast upregulation of RANKL, facilitating osteoclastogenesis [29]. Mitochondrial antiviral signal proteins (MAVS) also stimulate infected cells to secrete cytokines, activating the NF-κB and IRF3 pathways, which regulate the expression of type-I interferons. When bound to type-1 interferon receptors, these cytokines activate the JAK-STAT pathway, inhibiting pathogen replication and assembly due to a heightened expression of interferon-stimulated genes [30]. Type-I interferons such as INF-β are expressed in stromal cells, which are capable of differentiating into mature osteoblasts [31].
The combination of diabetic neuropathy, impaired perfusion, neutrophil dysfunction, and cytokine imbalance encourages infection development and progression, ischemic ulcers, or gangrene, which may culminate in amputation [11]. To avoid amputation and disease progression, a deeper understanding of pathogen involvement is necessary.
A wide variety of microorganisms may cause DFI, the most common of which is Staphylococcus aureus. The methicillin-resistant Staphylococcus aureus strain (MRSA) occurs in 16.78–30% of DFI cases, although this value varies geographically [32][33]. MRSA infection has been shown to have no impact on mortality but is correlated with an increased rate of hospitalization and higher risk of limb amputation [34]. Amputation is effective in preventing the spread of infection, and studies in the United Kingdom and Germany have found that the procedure increased the life expectancy by 2 years in 50% of the diabetic subjects studied [35][36]. Even so, only 56% of diabetic patients with ulcerative infections were found to survive 5 years after initial onset of the ulcer [36]. Altogether, this information highlights the need for improved ulcer prevention and the prompt diagnosis of DFI. Imaging may define the precise location of the infected bone and establish the border for amputation. Advanced molecular imaging methods may allow for early detection and verify the adequate response to therapy to avoid amputation.
References
- Lipsky, B.A.; Berendt, A.R.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; LeFrock, J.L.; Lew, D.P.; Mader, J.T.; Norden, C.; et al. Diagnosis and treatment of diabetic foot infections. Plast. Reconstr. Surg. 2006, 117, 212S–238S.
- Ponirakis, G.; Elhadd, T.; Chinnaiyan, S.; Dabbous, Z.; Siddiqui, M.; Al-Muhannadi, H.; Petropoulos, I.N.; Khan, A.; Ashawesh, K.A.E.; Dukhan, K.M.O.; et al. Prevalence and management of diabetic neuropathy in secondary care in Qatar. Diabetes Metab. Res. Rev. 2020, 36, e3286.
- Juster-Switlyk, K.; Smith, A.G. Updates in diabetic peripheral neuropathy. F1000Research 2016, 5, 738.
- Gregg, E.W.; Gu, Q.; Williams, D.; de Rekeneire, N.; Cheng, Y.J.; Geiss, L.; Engelgau, M. Prevalence of lower extremity diseases associated with normal glucose levels, impaired fasting glucose, and diabetes among U.S. adults aged 40 or older. Diabetes Res. Clin. Pract. 2007, 77, 485–488.
- Candrilli, S.D.; Davis, K.L.; Kan, H.J.; Lucero, M.A.; Rousculp, M.D. Prevalence and the associated burden of illness of symptoms of diabetic peripheral neuropathy and diabetic retinopathy. J. Diabetes Complicat. 2007, 21, 306–314.
- Bader, M.S. Diabetic foot infection. Am. Fam. Physician 2008, 78, 71–79.
- Ababneh, A.; Bakri, F.G.; Khader, Y.; Lazzarini, P.; Ajlouni, K. Prevalence and Associates of Foot Deformities among Patients with Diabetes in Jordan. Curr. Diabetes Rev. 2020, 16, 471–482.
- Lung, C.W.; Wu, F.L.; Zhang, K.; Liau, B.Y.; Townsend, R.; Jan, Y.K. Using elastographic ultrasound to assess plantar tissue stiffness after waking at different speeds and durations. Appl. Sci. 2020, 10, 7498.
- Wanzou, J.P.V.; Sekimpi, P.; Komagum, J.O.; Nakwagala, F.; Mwaka, E.S. Charcot arthropathy of the diabetic foot in a sub-Saharan tertiary hospital: A cross-sectional study. J. Foot Ankle Res. 2019, 12, 33.
- Wukich, D.K.; Sung, W. Charcot arthropathy of the foot and ankle: Modern concepts and management review. J. Diabetes Complicat. 2009, 23, 409–426.
- Bandyk, D.F. The diabetic foot: Pathophysiology, evaluation, and treatment. Semin. Vasc. Surg. 2018, 31, 43–48.
- Boulton, A.J.M. Diabetic neuropathy and foot complications. In Diabetes and the Nervous System; Elsevier: Amsterdam, The Netherlands, 2014; Volume 126, pp. 97–107. ISBN 1612764452.
- Giurato, L.; Meloni, M.; Izzo, V.; Uccioli, L. Osteomyelitis in diabetic foot: A comprehensive overview. World J. Diabetes 2017, 8, 135.
- Boulton, A.J.M.; Kirsner, R.S.; Vileikyte, L. Neuropathic diabetic foot ulcers. N. Engl. J. Med. 2004, 351, 48–55.
- Lázaro-Martínez, J.L.; Tardáguila-García, A.; García-Klepzig, J.L. Diagnostic and therapeutic update on diabetic foot osteomyelitis. Endocrinol. Diabetes Nutr. 2017, 64, 100–108.
- Lavery, L.A.; Crisologo, P.A.; La Fontaine, J.; Bhavan, K.; Oz, O.K.; Davis, K.E. Are We Misdiagnosing Diabetic Foot Osteomyelitis? Is the Gold Standard Gold? J. Foot Ankle Surg. 2019, 58, 713–716.
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.G.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. Executive summary: 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 2012, 54, 1679–1684.
- Lavery, L.A.; Ryan, E.C.; Ahn, J.; Crisologo, P.A.; Oz, O.K.; La Fontaine, J.; Wukich, D.K. The Infected Diabetic Foot: Re-Evaluating the IDSA Diabetic Foot Infection Classification. Clin. Infect. Dis. 2019, 70, 1573–1579.
- Lavery, L.A.; Armstrong, D.G.; Peters, E.J.G.; Lipsky, B.A. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: Reliable or relic? Diabetes Care 2007, 30, 270–274.
- Walton, D.M.; Minton, S.D.; Cook, A.D. The potential of transdermal nitric oxide treatment for diabetic peripheral neuropathy and diabetic foot ulcers. Diabetes Metab. Syndr. 2019, 13, 3053–3056.
- Petrova, N.L.; Petrov, P.K.; Edmonds, M.E.; Shanahan, C.M. Novel use of a Dektak 150 surface profiler unmasks differences in resorption pit profiles between control and Charcot patient osteoclasts. Calcif. Tissue Int. 2014, 94, 403–411.
- Ndip, A.; Williams, A.; Jude, E.B.; Serracino-Inglott, F.; Richardson, S.; Smyth, J.V.; Boulton, A.J.M.; Alexander, M.Y. The RANKL/RANK/OPG signaling pathway mediates medial arterial calcification in diabetic Charcot neuroarthropathy. Diabetes 2011, 60, 2187–2196.
- Rask-Madsen, C.; King, G.L. Vascular complications of diabetes: Mechanisms of injury and protective factors. Cell Metab. 2013, 17, 20–33.
- Burg, M.B.; Kador, P.F. Sorbitol, osmoregulation, and the complications of diabetes. J. Clin. Investig. 1988, 81, 635–640.
- Tanenberg, R.J.; Donofrio, P.D. Neuropathic Problems of the Lower Limbs in Diabetic Patients. In Levin and O’Neal’s The Diabetic Foot; Bowker, J.H., Pfeifer, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 33–74. ISBN 9780323041454.
- Hobizal, K.B.; Wukich, D.K. Diabetic foot infections: Current concept review. Diabet. Foot Ankle 2012, 3, 1849.
- Insuela, D.; Coutinho, D.; Martins, M.; Ferrero, M.; Carvalho, V. Neutrophil Function Impairment Is a Host Susceptibility Factor to Bacterial Infection in Diabetes. In Cells of the Immune System; IntechOpen: London, UK, 2020.
- Demirdal, T.; Sen, P. The significance of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and lymphocyte-monocyte ratio in predicting peripheral arterial disease, peripheral neuropathy, osteomyelitis and amputation in diabetic foot infection. Diabetes Res. Clin. Pract. 2018, 144, 118–125.
- Charles, J.F.; Nakamura, M.C. Bone and the innate immune system. Curr. Osteoporos. Rep. 2014, 12, 1–8.
- Seth, R.B.; Sun, L.; Ea, C.-K.; Chen, Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 2005, 122, 669–682.
- Gao, L.; Liesveld, J.; Anolik, J.; Mcdavid, A.; Looney, R.J. IFNβ signaling inhibits osteogenesis in human SLE bone marrow. Lupus 2020, 29, 1040–1049.
- Stacey, H.J.; Clements, C.S.; Welburn, S.C.; Jones, J.D. The prevalence of methicillin-resistant Staphylococcus aureus among diabetic patients: A meta-analysis. Acta Diabetol. 2019, 56, 907–921.
- Lavery, L.A.; La Fontaine, J.; Bhavan, K.; Kim, P.J.; Williams, J.R.; Hunt, N.A. Risk factors for methicillin-resistant Staphylococcus aureus in diabetic foot infections. Diabet. Foot Ankle 2014, 5.
- Saltoglu, N.; Ergonul, O.; Tulek, N.; Yemisen, M.; Kadanali, A.; Karagoz, G.; Batirel, A.; Ak, O.; Sonmezer, C.; Eraksoy, H.; et al. Influence of multidrug resistant organisms on the outcome of diabetic foot infection. Int. J. Infect. Dis. 2018, 70, 10–14.
- Icks, A.; Scheer, M.; Morbach, S.; Genz, J.; Haastert, B.; Giani, G.; Glaeske, G.; Hoffmann, F. Time-dependent impact of diabetes on mortality in patients after major lower extremity amputation: Survival in a population-based 5-year cohort in Germany. Diabetes Care 2011, 34, 1350–1354.
- Kerr, M. Foot Care for People with Diabetes: The Economic Case for Change. In Supporting, Improving, Caring; Insight Health Economics: London, UK, 2012.
More
Information
Subjects:
Surgery; Infectious Diseases
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Revisions:
3 times
(View History)
Update Date:
28 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




