Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Huajian Wang | + 2126 word(s) | 2126 | 2021-12-10 05:04:28 | | | |
| 2 | Yvaine Wei | -9 word(s) | 2117 | 2021-12-21 02:41:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, H. Multi-Element Imaging of the 1.4Ga Authigenic Siderite Crystal. Encyclopedia. Available online: https://encyclopedia.pub/entry/17323 (accessed on 02 March 2026).
Wang H. Multi-Element Imaging of the 1.4Ga Authigenic Siderite Crystal. Encyclopedia. Available at: https://encyclopedia.pub/entry/17323. Accessed March 02, 2026.
Wang, Huajian. "Multi-Element Imaging of the 1.4Ga Authigenic Siderite Crystal" Encyclopedia, https://encyclopedia.pub/entry/17323 (accessed March 02, 2026).
Wang, H. (2021, December 20). Multi-Element Imaging of the 1.4Ga Authigenic Siderite Crystal. In Encyclopedia. https://encyclopedia.pub/entry/17323
Wang, Huajian. "Multi-Element Imaging of the 1.4Ga Authigenic Siderite Crystal." Encyclopedia. Web. 20 December, 2021.
Copy Citation
The Al- and Fe-enriched zone in the core of siderite crystal might be an iron-bearing nucleus, and the formation of rim structure was related to the transition from a closed crystallization environment to a semi-closed growth environment. These results, combined with carbon isotope evidence from the siderites and surrounding shales, suggest that vigorous dissimilatory iron reduction that can provide Fe2+ and HCO3− to the pore water is a key factor to form the siderite-dominated Xiamaling IF.
siderite
iron
crystallization
multi-element imaging
LA-ICP-MS
Mesoproterozoic
Xiamaling Formation
dissimilatory iron reduction
1. Introduction
Most marine massive iron formations (IFs) were deposited during 3.0–1.8 Ga, with a brief return around 0.8 Ga [1]. During the one billion-year gap between 1.8 and 0.8 Ga, the IFs shortage is thought to be a result of significantly decreased Fe2+ concentration in the ocean, which was either substantially oxidized [2] or sulfurized [3]. Recent studies provide increasing evidence that ferruginous water was widespread in the deep ocean until 0.58 Ga [4][5]. Several Mesoproterozoic IFs have also been reported, such as the 1.40 Ga Xiamaling IF in North China [6][7] and the ~1.33 Ga Jingtieshan IF in Qilian [8]. However, different to the hematite- and magnetite-dominated 3.0−1.8 Ga IFs [9], the Xiamaling and Jingtieshan IFs are dominated by siderite [6][7][8]. In Australia, massive siderite deposits have also been found in the ~1.45 Ga Sherwin ironstone [10] and 2.5 Ga Hamersley IF [11]. Therefore, it is of great importance to explore the crystallization and formation mechanisms of siderite in the Precambrian ocean.
Siderite is a common Fe-bearing mineral, with FeCO3 as the main component. It is a calcite group of minerals, and its standard crystal form is mostly rhombohedral [12][13]. Siderite usually forms in specific conditions with either a hydrothermal origin or a diagenetic origin. Hydrothermal siderite is common in metallic veins and associated with sphalerite, barite, and other minerals [14][15]. Diagenetic siderite is mostly found in sedimentary rocks, such as clastic rocks [6], carbonate rocks [16], iron-beds [17], and coal [18]. Compared to the siderite formed in fresh water [19], the siderite in geological records is usually not pure, due to the substitution of Mn, Mg, and Ca for Fe [20][21]. Accompanied by substitution and growth, the primary siderite crystals can further transform into rhombohedral and/or massive siderite in the sediment [22].
In addition to the required water conditions, siderite formation also depends on the crystal nucleus and fluid saturation state [12]. Thus, authigenic siderite is mostly formed through biochemical reactions with reduction of iron-bearing minerals and oxidation of organic matter to provide Fe2+ and HCO3− [1][6][23]. The spheroidal siderite crystals formed in the ferruginous water column or pore water in response to the reactions of Fe-oxides and organic matters [21] have been considered as primary precipitates and precursors for rhombohedral and cemented diagenetic siderites in Precambrian rocks [22]. According to the diagenetic stages, the formation of authigenic siderite can be divided into three steps: (1) generation of colloidal aggregates with crystal nucleus; (2) generation of spherulites with poorly developed rim structures; (3) generation of oolites and rhombohedrons with well-developed rim structures [21]. The growth of siderite crystals always accompanies elemental substitution and mineral transformation, leading to varied morphologies and elemental compositions in different diagenetic stages. Therefore, petrological, mineralogical, and geochemical analyses of authigenic siderite are important for revealing the crystallization and formation mechanisms of siderite [24][25][26].
2. Geological Setting
The Xiamaling IF is distributed in the Yanliao Basin, which is located at the north margin of the North China Craton (Figure 1a–c). Paleo- and Mesoproterozoic sediments are found in the Yanliao Basin with a maximum thickness of ca. 8000 m [27]. The Xiamaling Formation is mainly composed of shale and siltstone and deposited over the carbonate rocks of the Tieling Formation with a parallel unconformity [28][29]. Two zircon U-Pb TIMS ages of 1384.4 ± 1.4 Ma and 1392.0 ± 1.0 Ma [28] and a zircon U-Pb LA-ICP-MS age of 1418 ± 14 Ma [29] have been obtained from the volcanic ash layers in the middle and bottom of the Xiamaling Formation, respectively (Figure 1d). In addition, abundant baddeleyite U-Pb ages of ca. 1320 Ma, obtained from the intruded gabbro-diabase sills in the Xiamaling sediments, are considered to be accompanied by a pre-magmatic uplift that started at ca. 1350 Ma [30], which further constrain the minimum age of Xiamaling Formation should be older than 1350 Ma.
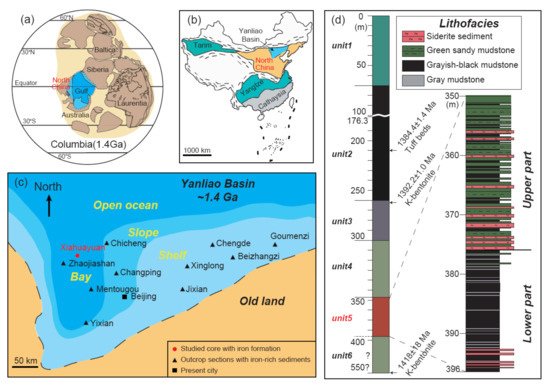
Figure 1. Location of the North China Craton at 1.4 Ga (a) and present (b). (c) Paleogeography of the Yanliao Basin at 1.4 Ga. (d) Stratigraphy of the Xiamaling iron formation defined as unit 5.
On the basis of the detailed geochemical and sedimentological investigation of the Xiamaling Formation at the Xiahuayuan section [6][28][31][32][33], a stratigraphic division scheme of six lithologic units in descending order was suggested. Among these, a 45 m-thickness iron-rich sediment at the Xiahuayuan section is defined as unit 5, which can be further divided to the lower part with grayish-black mudstone and the upper part with green sandy mudstone [6][34] (Figure 1d). The mainly Fe-bearing mineral in unit 5 is siderite with layer or nodule characteristics [6]. These iron-rich layers or nodules have also been found in other outcrop sections of the Xiamaling Formation (Figure 1c) [7][35][29][36], indicating a large-scale iron deposition event [6]. However, due to weathering, the Xiamaling IF in the outcrop are mostly red or yellowish-brown (Figure 2a–d). Fresh siderite samples can only be seen in the core (Figure 2e,f).

Figure 2. Photographs of the Xiamaling iron formation at the Xiahuayuan (a) and Zhaojiashan (b) sections. Photographs of the weathered siderite nodule (c) and layer (d) at the Xiahuayuan section. Photographs of the siderite nodules in the sandy mudstone (e) and gray shale (f).
3. Petrographic Characteristics of Siderite Crystals
Petrographic analysis reveals that most siderite crystals are subhedral to euhedral with a generally brown core and bright rims (Figure 3a,b). Most siderite crystals show high-grade white interference color. Here siderite is primarily present as two types. In one type, the samples are almost entirely composed of siderite minerals, which are ~50 μm subhedral (spherical) and densely packed (Figure 3a). In another, siderite crystals are discretely distributed in shale matrix (Figure 3b). This type of siderite is generally larger (~200 μm). In some well-preserved samples, euhedral rhombic crystals could be recognized (Figure 3b,c).

Figure 3. Photographs of the siderite crystals from the thin-sections of the pure siderite (a) and shale (b) samples from the Xiamaling Formation. (c) Photographs of an autogenic rhombohedral siderite. Photographs of the weathered iron (black spot) from the thin-sections (d,e) from outcrop.
However, for outcrop samples, due to surface weathering, siderite has been transformed into iron-oxides, such as limonite or goethite. These minerals are opaque under transmitted light. Though the boundaries between iron-rich minerals and matrix are still obvious (Figure 3d,e), those iron-bearing minerals have already lost their crystal characteristics (Figure 3e).
4. Multi-Element Imaging of a Siderite Crystal
Fe is the most abundant element in siderite with no obvious rim structure (Figure 4). The average Fe content of the crystal is 32.7%, corresponding to a FeCO3 purity of 72.5%. Compared with the surrounding shale, both Mn and Zn are enriched in the siderite with similar rim structures. From the core to edge, elements of Mn and Zn are synchronously enriched (rim 1), weakly enriched (rim 2), enriched (rim 3), and weakly enriched (rim 4) in turn (Figure 4). The wave features of the quantified contents of Zn and Mn in different rims varied over 50% between peaks and valleys (Figure 5).
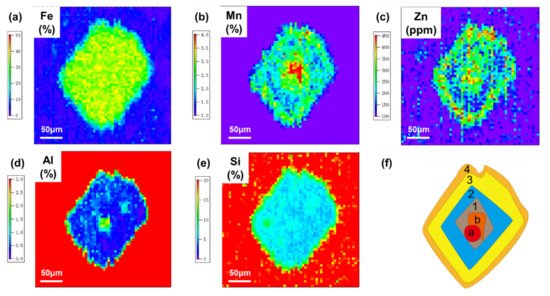
Figure 4. Multi-element imaging of an authigenic siderite crystal and its shale matrix obtained from the Laser ablation inductively coupled plasma mass spectrometry analysis. (a): Fe; (b): Mn; (c) Zn; (d): Al; (e): Si; (f) Structure diagram of the analyzed siderite crystal.
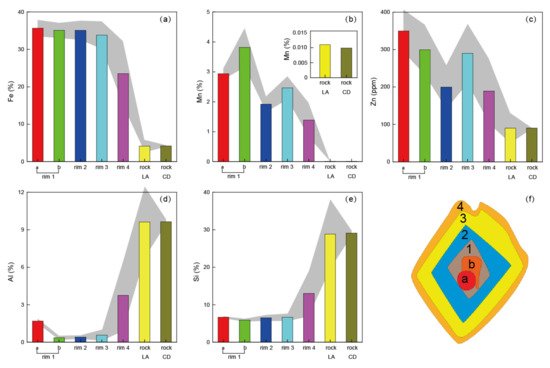
Figure 5. Element contents in different rims and zones, measured by LA-ICP-MS. Zones a and b are the rim 1 (core) of the crystal. The element contents in rock measured using chemical dissolution method (CD) were used to calculate the elements contents measured using LA-ICP-MS method (LA). The gray shadows indicate the standard division. (a): Fe; (b): Mn; (c) Zn; (d): Al; (e): Si; (f) Structure diagram of the analyzed siderite crystal.
Contrary to Mn and Zn, Al and Si exhibit depleted characteristics in the siderite crystal without obvious rims. However, in the core of crystal, there is a special zone (defined as zone a) with an Al content of 1.7%, higher than that in rim 2 (~0.4%) and rim 3 (~0.6%) (Figure 5). Next to zone a, there is another special zone (defined as zone b) with a Mn content of 3.8%, higher than that in rim 2 (~1.9%) and rim 3 (~2.5%). Thus, Al and Mn have further converse enrichment trends in zones a and b, while other elements do not show any differences (Figure 4 and Figure 5).
5. Supply of Fe2+ and HCO3− by Dissimilatory Iron Reduction
Another possible external source of seawater Fe2+ would be from the benthic shuttle transport from shelf [37] or terrestrial input [38]. Considering the widely distributed Xiamaling IF covering the whole Yanliao Basin, the benthic shuttle transport is also an unlikely contributor. Therefore, the most possible source should be biologically recycled continental Fe, which has been suggested as a major component in the 2.5 Ga Dales Gorge member IF [39]. Considering the still low oxidation levels in Mesoproterozoic atmosphere and ocean [40][41], the iron cycle in the ocean might be comparable to that of the terminal Archaean [34]. No matter which reason takes over, a ferruginous-dominated ocean during the Xiamaling Formation has already been confirmed [6][31][32][34][42][43]. Thus, Fe2+ would not be the limiting factor.
Since the Xiamaling siderites are mainly interbedded with shale or sandstone as layers or nodules, the capture of organic-sourced HCO3− by Fe2+ probably mainly occurred in pore water. Therefore, the siderite should be formed in a semi-closed to closed pore water conditions with Fe2+ and HCO3− supplies from seawater through DIR. A relatively closed pore water condition could also prevent the combination of HCO3− with Ca2+ to form calcite crystals, which, nevertheless, has a higher growth rate than siderite [19]. During the deposition period of the Xiamaling IF, DIR might have been quite vigorous, and could have reduced almost all the sinking iron-oxides into Fe2+ to form siderite later [6][7][34].
6. Fe-Bearing Mineral as a Nucleus
When the first two conditions have been met, the third requirement for siderite crystallization is a suitable nucleus, which can be a clastic particle (e.g., hematite, quartz, and feldspar) or even an organism remnant [21]. Because Al mainly exists in clay minerals, it can only exist before the siderite crystallization, and cannot be brought in during the crystallization process. However, this nucleus has an Fe content (~36%) similar to siderite, which can be determined to be an iron-bearing mineral. Robust DIR can almost completely reduce the hematite that has a much higher Fe content (70%) than siderite. This nucleus does not show any enrichment of Si, so the possibility of ferric silicate can also be ruled out. Therefore, it is most likely to be a residual siderite crystal with some clay contamination.
In fact, the occurrence of residual siderite crystal in 1.4 Ga sediments should be common. Firstly, the already found ~1.45 Ga Sherwin Ironstone in North Australia [10] and ~1.33 Ga Jingtieshan IF in Qilian [8] are siderite-dominated IF. Manganiferous dolostones are also present in the Tieling Formation (~1.44 Ga) and Gaoyuzhuang Formation (~1.58 Ga) [44][45][46]. These findings indicate that massive Fe and Mn deposits still existed in the Mesoproterozoic oceans but mainly in the form of carbonates. Secondly, there is a large unconformity between the Xiamaling and Tieling formations, indicating robust weathering and erosion of early sediments [29]. In different outcrop section, almost all the weathering crusts at the bottom of the Xiamaling Formation have high Fe contents [29][47]. The clastic materials such as sandstones and mudstones deposited above the crust are also rich in Fe [29][47]. Thus, it is possible that previously deposited carbonate minerals were uplifted to the surface by regional tectonic movement, and entered into the ocean again. The siderite nuclei provide mineral inclusion evidence for a possible siderite oxidation event at 1.4 Ga.
7. Growth of the Siderite Crystal
Different from the crystals in the siderite sample with a size around 50 μm, the siderite crystal in the shale sample can grow to 200 μm (Figure 4). Euhedral rhombohedral characteristic of the scanned siderite further indicates a weakly alkaline and closed water condition, which thereby allows the siderite to slowly grow as euhedral crystals [48]. However, unlike the uniform distribution of Fe on the crystal surface, the depletion of Mn and Zn in rim 2 might be caused by the failure to replenish them in a timely manner as the ions were deposited in a closed environment. In rim 3, the re-enriched Mn and Zn might be from the ferruginous pore water, which had limited Zn2+ and Mn2+. Then, the contents of Mn, Zn, and Fe underwent sharp decreases, while Al and Si contents increased conversely (Figure 4 and Figure 5), indicating a relatively open pore water with more clay and silicate minerals. In sum, the rim should be an indicator for the varied pore water conditions which changed from closed into semi-closed (Figure 6).
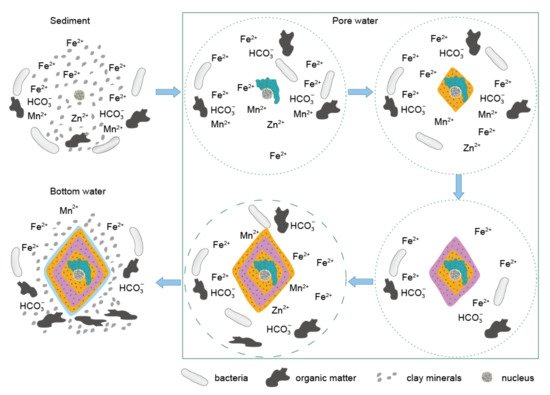
Figure 6. Conceptual model illustrating the crystallization and grow process of the studied siderite from the Xiamaling iron formation. The dot and dash circle in the pore water square represent closed and semi-closed condition, respectively.
References
- Konhauser, K.O.; Planavsky, N.J.; Hardisty, D.S.; Robbins, L.J.; Warchola, T.J.; Haugaard, R.; Lalonde, S.V.; Partin, C.A.; Oonk, P.B.H.; Tsikos, H.; et al. Iron formations: A global record of Neoarchaean to Palaeoproterozoic environmental history. Earth-Sci. Rev. 2017, 172, 140–177.
- Holland, H.D. Origins of breathable air. Nature 1990, 347, 17.
- Canfield, D.E. A new model for Proterozoic ocean chemistry. Nature 1998, 396, 450–453.
- Canfield, D.E.; Poulton, S.W.; Knoll, A.H.; Narbonne, G.M.; Ross, G.; Goldberg, T.; Strauss, H. Ferruginous conditions dominated later neoproterozoic deep-water chemistry. Science 2008, 321, 949–952.
- Poulton, S.W.; Canfield, D.E. Ferruginous conditions: A dominant feature of the ocean through earth’s history. Elements 2011, 7, 107–112.
- Canfield, D.E.; Zhang, S.C.; Wang, H.J.; Wang, X.M.; Zhao, W.Z.; Su, J.; Bjerrum, C.; Haxen, E.; Hammarlund, E. A Mesoproterozoic Iron Formation. Proc. Nat. Acad. Sci. USA 2018, 115, E3895–E3904.
- Tang, D.J.; Shi, X.Y.; Jiang, G.Q.; Wu, T.; Ma, J.B.; Zhou, X.Q. Stratiform siderites from the Mesoproterozoic Xiamaling Formation in North China: Genesis and environmental implications. Gondwana Res. 2018, 58, 1–15.
- Yang, X.Q.; Zhang, Z.H.; Santosh, M.; Duan, S.G.; Liang, T. Anoxic to suboxic Mesoproterozoic ocean: Evidence from iron isotope and geochemistry of siderite in the Banded Iron Formations from North Qilian, NW China. Precambrian Res. 2018, 307, 115–124.
- Bekker, A.; Slack, J.F.; Planavsky, N.; Krapež, B.; Hofmann, A.; Konhauser, K.O.; Rouxel, O.J. Iron formation: The sedimentary product of a complex interplay among mantle, tectonic, oceanic, and biospheric processes. Econ. Geol. 2010, 105, 467–508.
- Planavsky, N.J.; Reinhard, C.T.; Wang, X.L.; Thomson, D.; McGoldrick, P.; Rainbird, R.H.; Johnson, T.; Fischer, W.W.; Lyons, T.W. Low Mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science 2014, 346, 635–638.
- Czaja, A.D.; Johnson, C.M.; Beard, B.L.; Eigenbrode, J.L.; Freeman, K.H.; Yamaguchi, K.E. Iron and carbon isotope evidence for ecosystem and environmental diversity in the∼ 2.7 to 2.5 Ga Hamersley Province, Western Australia. Earth Planet. Sci. Lett. 2010, 292, 170–180.
- Mulders, J.J.; Tobler, D.J.; Oelkers, E.H. Siderite nucleation pathways as a function of aqueous solution saturation state at 25 °C. Chem. Geol. 2021, 559, 119947.
- Effenberger, H.; Mereiter, Κ.; Zemann, J. Crystal structure refinements of magnesite, calcite, rhodochrosite, siderite, smithonite, and dolomite, with discussion of some aspects of the stereochemistry of calcite type carbonates. Z. Krist. -Cryst. Mater. 1981, 156, 233–244.
- Wagner, T.; Cook, N. Sphalerite remobilization during multistage hydrothermal mineralization events—examples from siderite-Pb-Zn-Cu-Sb veins, Rheinisches Schiefergebirge, Germany. Miner. Petrol. 1998, 63, 223–241.
- Burisch, M.; Walter, B.F.; Gerdes, A.; Lanz, M.; Markl, G. Late-stage anhydrite-gypsum-siderite-dolomite-calcite assemblages record the transition from a deep to a shallow hydrothermal system in the Schwarzwald mining district, SW Germany. Geochim. Cosmochim. Acta 2018, 223, 259–278.
- Ellmies, R.; Voigtländer, G.; Germann, K.; Krupenin, M.; Moeller, P. Origin of giant stratabound deposits of magnesite and siderite in Riphean carbonate rocks of the Bashkir mega-anticline, western Urals. Geol. Rundsch. 1999, 87, 589–602.
- Heimann, A.; Johnson, C.M.; Beard, B.L.; Valley, J.W.; Roden, E.E.; Spicuzza, M.J.; Beukes, N.J. Fe, C, and O isotope compositions of banded iron formation carbonates demonstrate a major role for dissimilatory iron reduction in~2.5 Ga marine environments. Earth Planet. Sci. Lett. 2010, 294, 8–18.
- Li, Y.; Huang, W.; Jiu, B.; Sun, Q.; Che, Q. Modes of occurrence and origin of minerals in Permian coals from the Huainan coalfield, Anhui, China. Minerals 2020, 10, 399.
- Jiang, C.Z.; Tosca, N.J. Growth kinetics of siderite at 298.15 K and 1 bar. Geochim. Cosmochim. Acta 2020, 274, 97–117.
- Mozley, P.S. Relation between depositional environment and the elemental composition of early diagenetic siderite. Geology 1989, 17, 704–706.
- Vuillemin, A.; Wirth, R.; Kemnitz, H.; Schleicher, A.M.; Friese, A.; Bauer, K.W.; Simister, R.; Nomosatryo, S.; Ordoñez, L.; Ariztegui, D. Formation of diagenetic siderite in modern ferruginous sediments. Geology 2019, 47, 540–544.
- Köhler, I.; Konhauser, K.O.; Papineau, D.; Bekker, A.; Kappler, A. Biological carbon precursor to diagenetic siderite with spherical structures in iron formations. Nat. Commun. 2013, 4, 1741.
- Kamran, A.; Schneider, D.; Roddatis, V.; Thiel, V.; Hoppert, M. Formation of siderite in microbial microcosms derived from a marine sediment. Geomicrobio. J. 2020, 37, 475–485.
- Sengupta, R.; Tosca, N.J.; Robinson, S.A. Geochemical controls on the elemental composition of siderite: Implications for palaeo-environmental reconstructions. Geochim. Cosmochim. Acta 2020, 271, 1–15.
- Pe-Piper, G.; Piper, D.J. Significance of the chemistry and morphology of diagenetic siderite in clastic rocks of the Mesozoic Scotian Basin. Sedimentology 2020, 67, 782–809.
- Koo, T.-h.; Kim, J. Controls on the formation and stability of Siderite (FeCO3) and Chukanovite (Fe2(CO3)(OH)2) in reducing environment. Minerals 2020, 10, 156.
- Meng, Q.R.; Wei, H.H.; Qu, Y.Q.; Ma, S.X. Stratigraphic and sedimentary records of the rift to drift evolution of the northern North China craton at the Paleo-to Mesoproterozoic transition. Gondwana Res. 2011, 20, 205–218.
- Zhang, S.C.; Wang, X.M.; Hammarlund, E.U.; Wang, H.J.; Costa, M.M.; Bjerrum, C.J.; Connelly, J.N.; Zhang, B.M.; Bian, L.Z.; Canfield, D.E. Orbital forcing of climate 1.4 billion years ago. Proc. Nat. Acad. Sci. USA 2015, 112, E1406–E1413.
- Lyu, D.; Deng, Y.; Wang, H.; Zhang, F.; Ren, R.; Gao, Z.; Zhou, C.; Luo, Z.; Wang, X.; Bi, L.; et al. Using cyclostratigraphic evidence to define the unconformity caused by the Mesoproterozoic Qinyu Uplift in the North China Craton. J. Asian Earth Sci. 2021, 206, 104608.
- Zhang, S.H.; Zhao, Y.; Li, X.H.; Ernst, R.E.; Yang, Z.Y. The 1.33-1.30 Ga Yanliao large igneous province in the North China Craton: Implications for reconstruction of the Nuna (Columbia) supercontinent, and specifically with the North Australian Craton. Earth Planet. Sci. Lett. 2017, 465, 112–125.
- Zhang, S.C.; Wang, X.M.; Wang, H.J.; Bjerrum, C.J.; Hammarlund, E.U.; Haxen, E.R.; Wen, H.J.; Ye, Y.T.; Canfield, D.E. Paleoenvironmental proxies and what the Xiamaling Formation tells us about the mid-Proterozoic ocean. Geobiology 2019, 17, 225–246.
- Wang, X.M.; Zhang, S.C.; Wang, H.J.; Bjerrum, C.J.; Hammarlund, E.U.; Haxen, E.R.; Su, J.; Wang, Y.; Canfield, D.E. Oxygen, climate and the chemical evolution of a 1400-million-year old tropical marine setting. Am. J. Sci. 2017, 317, 861–900.
- Zhang, S.C.; Wang, X.M.; Wang, H.J.; Bjerrum, C.J.; Hammarlund, E.U.; Costa, M.M.; Connelly, J.N.; Zhang, B.M.; Su, J.; Canfield, D.E. Sufficient oxygen for animal respiration 1,400 million years ago. Proc. Nat. Acad. Sci. USA 2016, 113, 1731–1736.
- Wang, H.J.; Ye, Y.T.; Deng, Y.; Wang, X.M.; Hammarlund, E.U.; Fan, H.F.; Canfield, D.E.; Zhang, S.C. Iron isotope evidence for a dynamic marine Fe cycle 1.4 Ga. Geochem. Perspect. Let. 2021. under review.
- Zhang, K.; Zhu, X.K. Basic geological characteristics of the siderite-rich strata in the Xiamaling Formation, Jixian County. Acta Petrol. Miner. 2013, 32, 529–537. (In Chinese)
- Fan, W.B. Geological features and research progress of the Mesoproterozoic Xiamaling Formation in the North China Craton: A review after nearly one hundred years of study. Geol. Rev. 2015, 61, 1383–1406. (In Chinese)
- Lyons, T.W.; Severmann, S. A critical look at iron paleoredox proxies: New insights from modern euxinic marine basins. Geochim. Cosmochim. Acta 2006, 70, 5698–5722.
- Holland, H.D. The oceans; a possible source of iron in iron-formations. Econ. Geol. 1973, 68, 1169–1172.
- Li, W.Q.; Beard, B.L.; Johnson, C.M. Biologically recycled continental iron is a major component in banded iron formations. Proc. Nat. Acad. Sci. USA 2015, 112, 8193–8198.
- Zhang, S.; Wang, H.; Wang, X.; Ye, Y. The Mesoproterozoic Oxygenation Event. Sci. China Earth Sci. 2021, 64, 1–26.
- Canfield, D.E.; van Zuilen, M.A.; Nabhan, S.; Bjerrum, C.J.; Zhang, S.; Wang, H.; Wang, X. Petrographic carbon in ancient sediments constrains Proterozoic Era atmospheric oxygen levels. Proc. Nat. Acad. Sci. USA 2021, 118, e2101544118.
- Wang, H.; Zhang, Z.; Li, C.; Algeo, T.J.; Cheng, M.; Wang, W. Spatiotemporal redox heterogeneity and transient marine shelf oxygenation in the Mesoproterozoic ocean. Geochim. Cosmochim. Acta 2020, 270, 201–217.
- Tang, D.; Ma, J.; Shl, X.; Lechte, M.; Zhou, X. The formation of marine red beds and iron cycling on the Mesoproterozoic North China Platform. Am. Miner. 2020, 105, 1412–1423.
- Fang, H.; Tang, D.; Shi, X.; Lechte, M.; Yu, W. Manganese-rich deposits in the Mesoproterozoic Gaoyuzhuang Formation (ca. 1.58 Ga), North China Platform: Genesis and paleoenvironmental implications. Palaeogeogr. Palaeocl. 2020, 559, 109966.
- Ye, L.; Fan, D.; Yang, P. Characteristics of manganese ore deposits in China. Ore. Geol. Rev. 1988, 4, 99–113.
- Mei, M.; Yang, F.; Gao, J.; Meng, Q. Glauconites formed in the high-energy shallow-marine environment of the Late Mesoproterozoic: Case study from Tieling Formation at Jixian Section in Tianjin, North China. Front. Earth Sci. 2008, 15, 146–158.
- Qiao, X. Investigation on stratigraphy of the Qingbaikou Group of the Yanshan Mountains, North China. Chinese J. Geol. 1976, 3, 246–264. (In Chinese)
- Weibel, R.; Lindström, S.; Pedersen, G.; Johansson, L.; Dybkjaer, K.; Whitehouse, M.; Boyce, A.; Leng, M. Groundwater table fluctuations recorded in zonation of microbial siderites from end-Triassic strata. Sediment. Geol. 2016, 342, 47–65.
More
Information
Subjects:
Geochemistry & Geophysics
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
21 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





