
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | En-Qin Xia | + 2237 word(s) | 2237 | 2021-09-26 05:02:28 | | | |
| 2 | Catherine Yang | Meta information modification | 2237 | 2021-12-20 05:23:20 | | |
Video Upload Options
Aging is a biological process that occurs under normal conditions and in several chronic degenerative diseases. Bioactive natural peptides have been shown to improve the effects of aging in cell and animal models and in clinical trials. However, few reports delve into the enormous diversity of peptides from marine organisms.
1. Introduction
In recent history, human life expectancy has continuously risen, and the proportion of the elderly population has sharply increased with the improvement of medical technology. Dysfunction of normal biological processes accompanies aging, and the acceleration of molecular damage and dysfunction in cells, tissues, and organs eventually leads to aging-related diseases, such as cardiovascular diseases, nutritional metabolic diseases, and cognitive disorders [1][2]. However, the aging process is adjustable, albeit irreversible, and scientific approaches can regulate the speed of aging and promote good health [3]. Therefore, development of efficient strategies to manage aging is critical for community health. In recent years, a growing body of work has aimed to test several hypotheses related to the molecular mechanisms that play critical roles in determining longevity. Among them, reactive oxygen species (ROS) inducing oxidative stress is considered one of the primary contributors to aging [4]. Hence, exploration of substances with antioxidant activity is an important strategy for the management of aging [5][6]. Many kinds of anti-aging drugs have been synthesized and tested in clinical trials, but many obstacles prevent drug development, including the adverse reactions of the human body to synthesized drugs. Therefore, natural active substances with high efficiency and low side effects are important alternatives for combating aging [6].
Recently, natural bioactive peptides have attracted special attention. Biologically active peptides with anticancer, anticoagulant, antidiabetic, antihypertensive, antimicrobial, antioxidant, and cholesterol-lowering properties have been reported in the biomedicine and pharmaceutical biotechnology literature [7]. Some of these compounds also exhibit notable antiaging activity in vitro or in vivo. Presently, potential bioactive peptides are mainly isolated and identified in different food products like milk, soy, rice, meat, fish, lobsters, crabs, and shrimp [8]. Some bioactive peptides are extremely safe, easily absorbed, and have no toxic side effects [9][10]. Moreover, because of their hypo-allergenicity characteristics, bioactive peptides have been explored as a method to treat patients with food allergies [11]. Therefore, bioactive peptides should be considered as excellent alternatives for anti-aging treatment.
Marine organisms contain high-quality functional protein or peptides with diverse molecular structures, and are an important source of new compounds, especially for China with its 18,000 km coastline, compared to terrestrial resources [12]. Presently, excluding various fish that are widely used as food materials, there is only limited use of marine protein resources from other species, such as sea cucumber, sea urchin, mussels, and several kinds of algae. Some marine bioactive peptides regulate free radical homeostasis in vitro and in vivo, and can have antiaging effects in cell and animal models and in human clinical trials [13][14][15][16]. Marine natural bioactive peptides have also been used in cosmeceutical skin products as antiaging agents [17][18][19].
2. Antiaging Activity of Peptides from the Ocean
ROS are highly reactive substances, and overproduction of ROS can result in DNA mutation, lipid, and protein dysfunction, and, ultimately, aging [20]. Thus, the main protective strategy to slow aging is to scavenge the excessive ROS by providing antioxidant agents, as well restoring the antioxidant defense system in cells. In the human body, the antioxidant defense system consists mainly of antioxidant peptides, including carnosine, glutathione, and antioxidant enzymes, including superoxide dismutase (SOD), glutathione oxidase (GHS-Px) and catalase (CAT).
3. The Mechanisms Underlying Antiaging Activity
Marine antiaging peptides have been shown to act in several different ways, including scavenging free radical capacities in vitro, inhibiting cell apoptosis, prolonging the life span of fruit flies and nematodes, and ameliorating D-galactose levels that induce aging in mice, and have shown promise as antiaging agents in human clinical trials.
Antioxidant enzymes are an important target of peptides in cells and organisms. The underlying mechanisms involved include regulation of the classical antioxidant gene Klotho and the Keap1/nuclear fac-tor erythroid 2-related factor 2- antioxidant responsive element (Nrf2-ARE) signaling pathway. In addition, marine peptides can regulate DAF-16/FOXO-SOD-3 expression, i.e., a forkhead transcription factor/forkhead transcription factors of the O class, which can enhance antioxidant enzyme activity.
The antiaging properties of the Leu-Cys-Gly-Glu-Cys peptide have been verified in healthy mouse models. After treatment with the peptides, mice display a healthier status based on a series of body indices, such as increased body weight and decreased liver and brain indices. Based on the analysis of liver and serum samples, the peptide was found to increase the activity of serum GSH-Px and SOD and decrease levels of MDA. Additionally, the transcription levels of Keap1 and Nrf2 were significantly downregulated in the liver and brain. These results confirmed that marine bioactive peptides can be used as potential regulators of aging in cells and mice, extending the earlier findings that showed it can scavenge free radicals in vitro. These results also highlight the importance of the Keap1/Nrf2-ARE pathway in antiaging [28].
As summarized in Table 1 , marine antioxidant peptides have potential as antiaging agents because they can protect cells from oxidative stress by directly scavenging excess free radicals. In addition, marine peptides can inhibit apoptosis via two pathways: the p53-Bax/Bcl-2 (BCL2-associated X protein/B lymphoblastic cells) pathway and the Mammalian target of rapamycin complex 1 (mTORC1)-mTOR-Bcl/Bax pathway.
Table 1. Marine antiaging peptides and their free radical scavenging activity in vitro.
| Source | Sequences | Activities (EC50) | Ref. |
|---|---|---|---|
| Microorganism | |||
| Chlorella vulgaris | favourzyme hydrolysates | Superoxide3 (0.323 mg/mL), Hydroxyl2 (0.139 mg/mL) |
[29] |
| Spirulina sp. | Thr-Met-Glu-Pro-Gly-Lys-Pro | Inhibition of ROS production | [30] |
| Dunaliella salina | Ile-Leu-Thr-Lys-Ala-Ala-Ile-Glu-Gly-Lys Ile-Ile-Tyr-Phe-Gln-Gly-Lys Asn-Asp-Pro-Ser-Thr-Val-Lys Thr-Val-Arg-Pro-Pro-Gln-Arg |
DPPH1 | [31] |
| Penicillium brevicompactum | N-cinnamoyl tripeptide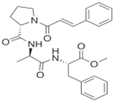 |
Hydroxyl2 (equivalent to that of quercetin at 0.1 mM) | [32] |
| Kocuria marina | Phe-Glu, Asp-Ile, Ser-Ser-Gln, Leu-Glu | DPPH1 (0.24 mg/mL) | [33] |
| Marine invertebrates | |||
| Neptunea arthritica cumingii | Tyr-Ser-Gln-Leu-Glu-Asn-Glu-Phe-Asp-Arg | DPPH1 (0.77 mM) | [34] |
| Tyr-Ile-Ala-Glu-Asp-Ala-Glu-Arg | DPPH1 (1.04 mM) | ||
| Tergillarca granosa | Glu-Met-Gly-Pro-Ala | DPPH1 (0.53 ± 0.02 mg/mL), Hydroxyl2 (0.47 ± 0.03 mg/mL), Superoxide3 (0.75 ± 0.04 mg/mL), ABTS4(0.96 ± 0.08 mg/mL), Inhibition of lipid peroxidation | [35] |
| Trp-Pro-Pro-Asp | DPPH1 (0.36 ± 0.02 mg/mL), Hydroxyl1 (0.38 ± 0.04 mg/mL), Superoxide3 (0.46 ± 0.05 mg/mL), ABTS4 (0.54 ± 0.03 mg/mL), Inhibition of lipid peroxidation | ||
| Brachionus rotundiformis | Leu-Leu-Gly-Pro-Gly-Leu-Thr-Asn-His-Ala, | DPPH1 (189.8 µM) | [36] |
| Asp-Leu-Gly-Leu-Gly-Leu-Pro-Gly-Ala-His | DPPH1 (167.7 µM) | ||
| Fish | |||
| Muscle of Scomberomorous niphonius | Pro-Glu-Leu-Asp-Trp | DPPH1 (1.53 mg/mL), Hydroxyl2 (1.12 mg/mL), Superoxide2 (0.85 mg/mL), Inhibition of lipid peroxidation, Protection of plasmid DNA | [37] |
| Trp-Pro-Asp-His-Trp | DPPH1 (0.70 mg/mL). Hydroxyl2 (0.38 mg/mL) Superoxide3 (0.49 mg/mL). Inhibition of lipid peroxidation, Protect plasmid DNA. | ||
| Phe-Gly-Tyr-Asp-Trp-Trp | DPPH1 (0.53 mg/mL), Hydroxyl2 (0.26 mg/mL), Superoxide3 (0.34 mg/mL). Inhibition of lipid peroxidation, | ||
| Tyr-Leu-His-Phe-Trp | DPPH1 (0.97 mg/mL), Hydroxyl2 (0.67 mg/mL), Superoxide3 (1.37 mg/mL), Inhibit lipid peroxidation. | ||
| Skin of Scomberomorous niphonius | Pro-Phe-Gly-Pro-Asp | DPPH1 (0.80 mg/mL), Hydroxyl2 (0.81 mg/mL), Superoxide3 (0.91 mg/mL, ABTS4 (0.86 mg/mL), FRAP and Inhibition of lipid peroxidation | [38] |
| Pro-Tyr-Gly-Ala-Lys-Gly | DPPH1 (3.02 mg/mL), Hydroxyl2 (0.66 mg/mL), Superoxide3 (0.80 mg/mL), ABTS4 (1.07 mg/mL), FRAP and inhibit lipid peroxidation | ||
| Tyr-Gly-Pro-Met | DPPH1 (0.72 mg/mL), Hydroxyl2 (0.88 mg/mL), Superoxide3 (0.73 mg/mL), ABTS4 (0.82 mg/mL), FRAP and inhibit lipid peroxidation | ||
| Cartilage of Dasyatis akajei | Ile-Glu-Glu-Glu-Gln | DPPH1 (4.61 mg/mL), Hydroxyl2 (0.77 mg/mL), Superoxide3 (0.08 mg/mL), ABTS4 (0.15 mg/mL). | [39] |
| Ile-Glu-Pro-His | DPPH1 (1.90 mg/mL,), Hydroxyl2 (0.46 mg/mL), Superoxide3 (0.17 mg/mL), ABTS4 (0.11 mg/mL), Lipid peroxidation inhibition activity. | ||
| Leu-Glu-Glu-Glu-Glu | DPPH1 (3.69 mg/mL), Hydroxyl2 (0.70 mg/mL), Superoxide3 (0.15 mg/mL), ABTS4 (0.19 mg/mL), Fe2+-chelating ability. | ||
| Val-Pro-Arg | DPPH1 (4.01 mg/mL), Hydroxyl2 (1.30 mg/mL), Superoxide3 (0.16 mg/mL), ABTS4 (0.18 mg/mL). | ||
| Head of Katsuwonus pelamis | Trp-Met-Gly-Pro-Tyr | DPPH1 (0.33 mg/mL), Hydroxyl2 (0.43 mg/mL), Superoxide3 (0.38 mg/mL), FRAP and lipid peroxidation inhibition. | [40] |
| Trp-Met-Phe-Asp-Trp | DPPH1 (0.31 mg/mL), Hydroxyl2 (0.30 mg/mL), Superoxide3 (0.56 mg/mL), FRAP and lipid peroxidation inhibition. | ||
| Glu-Met-Gly-Pro-Ala | DPPH1 (0.46 mg/mL), Hydroxyl2 (0.52 mg/mL), Superoxide3 (0.71 mg/mL), FRAP and lipid peroxidation inhibition. | ||
| Salmon gelatin | Gly-Gly-Pro-Ala-Gly-Pro-Ala-Val, Gly-Pro-Val-Ala, Pro-Pro and Gly-Phe | Oxygen radical absorbance capacity (ORAC, 540.94 ± 9.57 µmol TE/g d.w.) | [41] |
| Pacific cod skin gelatin | Leu-Leu-Met-Leu-Asp-Asn-Asp-Leu-Pro-Pro | Scavenging the intracellular ROS | [42] |
| Jumbo squid (Dosidicus gigas, squid) skin gelatin | Phe-Asp-Ser-Gly-Pro-Ala-Gly-Val-Leu Asn-Gly-Pro-Leu-Gin-Ala-Gly-Gln-Pro-Gly-Glu-Arg |
Inhibition of oxidant stress; Lipid peroxidation inhibition (>Vit. E). | [43] |
| Whole body of Parastromateus niger | Ala-Met-Thr-Gly-Leu-Glu-Ala | DPPH1 (54%), Metal chelating (78.6%) at 1 mg/mL | [44] |
| Smooth hound viscera (sharks) | Protein hydrate containing Gly, Glx, Lys, Asx, Arg, Pro and Ala | DPPH1, Inhibition of linoleic acid oxidation, Hydroxyl2. | [45] |
| Hoki (Johnius belengerii) frame | Glu-Ser-Thr-Val-Pro-Glu-Arg-Thr-His-Pro-Ala-Cys-Pro-Asp-Phe-Asn | DPPH1 (41.37 µM), Hydroxyl2 (17.77 µM), Peroxyl radical scavenging (18.99 µM), Superoxide3 (172.10 µM). | [46] |
| Limanda aspera frame | Arg-Pro-Asp-Phe-Asp-Leu-Glu-Pro-Pro-Tyr | Inhibition of linoleic acid autoxidation | [47] |
| Tuna backbone | Val-lys-Ala-Gly-Phe-Ala-Trp-Thr-Ala-Asn-Gln-Gln-Leu-Ser | Inhibited lipid peroxidation, Quenched free radicals (DPPH, hydroxyl and superoxide) | [48] |
| Frame of Theragra chalcogramma | Leu-Pro-His-Ser-Gly-Tyr | Hydroxyl2 (35% at 53.6 µM) | [49] |
Note: DPPH1-DPPH radical scavenging capacity, Hydroxyl2-Hydroxyl radical scavenging capacity, Superoxide3-Superoxide anion radical scavenging capacity, ABTS4-ABTS cation radical scavenging capacity.
As shown in Table 2 , recent investigations have focused on the impact of marine peptides on gut microbiota. The test substances have included protein hydrolysates, polypeptide fractions, and pure antioxidant peptides. Significant positive effects have been observed in many assays using peptides from microalgea, invertebrates, and byproducts of the fishing industry, such as the milt, roe and viscera of fish. Even glycosylated fish has been shown to have positive results. The balance of gut microbiota populations significantly improves not only in healthy mice or rats, but also in unhealthy ones subject to fatigue, alcohol-induced injury, and high-fat diet (HFD). In addition, a peptide combined with calcium significantly increases the abundance of beneficial gut microbes, including Firmicutes and Lactobacillus , in low-calcium diet-fed rats. Calcium absorption also increased in these animals.
Table 2. Regulation of gut microbiota by marine peptides.
| Marine Peptides | Test Animal | Improvement of Gut Microbiota | Ref. |
|---|---|---|---|
| Spirulina Phycocianin (Microalgae) | Mice | Increase the relative abundance of Bacteroidetes and Actinobacteria | [50] |
| Glycosylated fish protein | Mice | Increase the abundance of Allobaculum, Akkermansia, Lactobacillus animalis | [51] |
| Walleye Pollock skin | Mice | Upregulation relative abundance of Lactobacillus and Akkermansia, downregulation the abundance of bacteria associated with intestinal inflammation | [52] |
| Skin collagen peptide of Salmon salar and Tilapia nilotica | Male rats | Increased abundance of Lactobacillus | [53] |
| Herring milt hydrolysate (protein: 47–94%) | Mice | Maintain abundant of Lactobacillus decrease metabolites associated with obesity and inflammatory disease |
[54] |
| Peptides from tuna roe | Mice | Short-chain fatty acids production in feces and modulating gut microbiota composition | [28] |
| Abalone viscera | Alcohol induced injured mice | Increase in diversity index and the number of Bacilli (class), Lactobacillales (order), Lactobacillaceae (family), and Lactobacillus (genus) levels | [55] |
| Spirulina platensis protease hydrolyzate | High-fat diet (HFD)-fed rats | Enriched the abundance of gut beneficial bacteria | [56] |
| Chlorella pyrenoidosa protein hydrolysate-calcium chelate | Low-calcium diet-fed rats | Improving the abundances of Firmicutes and Lactobacillus | [57] |
| Oyster polypeptide (OP) fraction | Exhaustive fatigue mice | regulate the abundance of gut microbiota and maintain its balance | [58] |
4. Conclusions and Perspectives
The potential antiaging activities of natural peptides from underused marine organisms have been investigated in vitro, in cells, in animal models and in human clinical trials. These peptides act using the following molecular mechanisms: free radical scavenging, enhancing oxidase activity, protecting mitochondria, downregulating the apoptotic pathway, inhibiting MMP-1 expression, and restoring intestinal homeostasis. These results suggest that underused marine peptides have great potential as functional food and cosmetic ingredients for antiaging purposes. However, presently, there are three major outstanding issues. First, relevant research reports in the Web of Science database are few, and not enough animal assays have reported. We therefore focused on in vitro assays for this review. Second, in the limited literature, very few marine species have been explored, and vast marine protein resources are still underutilized. Third, apart from several applications in skin aging, there is a dearth of information about marine peptides in human clinical trials related to antiaging. This lack of literature makes it difficult to apply and transform these peptides for the market. To address these issues, further exploration of the abundant underused marine peptide sources is required.
References
- Mitchell, S.J.; Scheibye-Knudsen, M.; Longo, D.L.; de Cabo, R. Animal models of aging research: Implications for human aging and age-related diseases. Annu. Rev. Anim. Biosci. 2015, 3, 283–303.
- Xia, E.; Zhu, S.; He, M.; Luo, F.; Fu, C.; Zou, T. Marine peptides as potential agents for the management of Type 2 Diabetes Mellitus—A prospect. Mar. Drugs 2017, 15, 88.
- Podhorecka, M.; Ibanez, B.; Dmoszyńska, A. Metformin—Its potential anti-cancer and anti-aging effects. Postep. Hig. Med. Dosw. (Online) 2017, 71, 170–175.
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300.
- Lara, J.; Sherratt, M.J.; Rees, M. Aging and anti-aging. Maturitas 2016, 93, 1–3.
- Son, D.H.; Park, W.J.; Lee, Y.J. Recent advances in anti-aging medicine. Korean J. Fam. Med. 2019, 40, 289–296.
- Jin, Q.; Peng, D.; Zheng, Z. Advances in extracting and understanding the bioactivities of marine organism peptides: A review. J. Food Process Pres. 2021, e15602.
- Dhaval, A.; Yadav, N.; Purwar, S. Potential applications of food derived bioactive peptides in management of health. Int. J. Pept. Res. Ther. 2016, 22, 377–398.
- Beltrán-Barrientos, L.M.; García, H.S.; Torres-Llanez, M.J.; González-Córdova, A.F.; Hernández-Mendoza, A.; Vallejo-Cordoba, B. Safety of milk-derived bioactive peptides. Int. J. Dairy Technol. 2017, 70, 16–22.
- Schaafsma, G. Safety of protein hydrolysates, fractions thereof and bioactive peptides in human nutrition. Eur. J. Clin. Nutr. 2009, 63, 1161.
- Clemente, A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci. Technol. 2000, 11, 254–262.
- Chai, M.; Ye, Y.; Chen, V. Separation and concentration of milk proteins with a submerged membrane vibrational system. J. Membr. Sci. 2017, 524, 305–314.
- Jo, C.; Khan, F.F.; Khan, M.I.; Iqbal, J. Marine bioactive peptides: Types, structures, and physiological functions. Food Rev. Int. 2017, 33, 44–61.
- Edgar, S.; Hopley, B.; Genovese, L.; Sibilla, S.; Laight, D.; Shute, J. Effects of collagen-derived bioactive peptides and natural antioxidant compounds on proliferation and matrix protein synthesis by cultured normal human dermal fibroblasts. Sci. Rep. 2018, 8, 10474.
- Ito, N.; Seki, S.; Ueda, F. Effects of composite supplement containing collagen peptide and ornithine on skin conditions and plasma IGF-1 levels-A randomized, double-blind, placebo-controlled trial. Mar. Drugs 2018, 16, 482.
- Zhou, T.; Wang, N.; Xue, Y.; Ding, T.; Liu, X.; Mo, X.; Sun, J. Development of Biomimetic Tilapia collagen nanofibers for skin regeneration through inducing keratinocytes differentiation and collagen synthesis of dermal fibroblasts. ACS Appl. Mater. Inter. 2015, 7, 3253–3262.
- Lima, T.N.; Moraes, C.A.P. Bioactive peptides: Applications and relevance for cosmeceuticals. Cosmetics 2018, 5, 21.
- Aguilar-Toalá, J.E.; Hernández-Mendoza, A.A.; González-Córdov, F.; Vallejo-Cordob, B.A.; Liceaga, M. Potential role of natural bioactive peptides for development of cosmeceutical skin products. Peptides 2019, 122, 170170.
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Marine fish proteins and peptides for cosmeceuticals: A review. Mar. Drugs 2017, 15, 143.
- Schulz, I.; Mahler, H.C.; Boiteux, S.; Epe, B. Oxidative DNA base damage induced by singlet oxygen and photosensitization: Recognition by repair endonucleases and mutagenicity. Mutat. Res. 2000, 461, 145–156.
- Schmedes, M.; Brejnrod, A.D.; Aadland, E.K.; Kiilerich, P.; Kristiansen, K.; Jacques, H.; Lavigne, C.; Graff, I.E.; Eng, Ø.; Holthe, A.; et al. The effect of lean-seafood and non-seafood diets on fecal metabolites and gut microbiome: Results from a randomized crossover intervention study. Mol. Nutr. Food Res. 2019, 63, e1700976.
- Han, S.B.; Won, B.; Yang, S.C.; Kim, D.H. Asterias pectinifera derived collagen peptide-encapsulating elastic nanoliposomes for the cosmetic application. J. Ind. Eng. Chem. 2021, 98, 289–297.
- Wang, Y.; Gagnon, J.; Nair, S.; Sha, S. Herring milt protein hydrolysate improves iInsulin resistance in high-fat-diet-induced obese male C57BL/6J mice. Mar. Drugs 2019, 17, 456.
- Jang, J.; Kim, M.; Nam, G.-H.; Kang, S.; Lee, K.-Y.; Park, Y.-J. Antiaging activity of peptide identified from fermented Trapa Japonica fruit extract in human dermal fibroblasts. Evid.-Based Compl. Alt. Med. 2020, 2020, 1–8.
- DeBacker, C.M.; Putterman, A.M.; Zhou, L.; Holck, D.E.E.; Dutton, J.J. Age-related changes in type-I collagen synthesis in human eyelid skin. Ophthal. Plast. Recons. Surg. 1998, 14, 13–16.
- Sibilla, S.; Godfrey, M.; Brewer, S.; Budh-Raja, A.; Genovese, L. An overview of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: Scientific background and clinical studies. Open Nutraceut. J. 2015, 8, 29–42.
- Fan, J.; Zhuang, Y.; Li, B. Effects of collagen and collagen hydrolysate from jellyfish umbrella on histological and immunity changes of mice photoaging. Nutrients 2013, 5, 223–233.
- Han, J.; Huang, Z.; Tang, S.; Lu, C.; Wan, H.; Zhou, J.; Li, Y.; Ming, T.; Jim Wang, Z.; Su, X. The novel peptides ICRD and LCGEC screened from tuna roe show antioxidative activity via Keap1/Nrf2-ARE pathway regulation and gut microbiota modulation. Food Chem. 2020, 327, 127094.
- Zhang, Y.; Jiang, W.; Hao, X.; Tan, J.; Wang, W.; Yu, M.; Zhang, G.; Zhang, Y. Preparation of the Enzymatic hydrolysates from Chlorella vulgaris protein and assessment of their antioxidant potential using Caenorhabditis elegans. Mol. Biotechnol. 2021, 1–9.
- Heo, S.Y.; Ko, S.C.; Kim, C.S.; Oh, G.W.; Ryu, B.; Qian, Z.J.; Kim, G.; Park, W.S.; Choi, I.W.; Phan, T.T.; et al. A heptameric peptide purified from Spirulina sp. gastrointestinal hydrolysate inhibits angiotensin I-converting enzyme- and angiotensin II-induced vascular dysfunction in human endothelial cells. Int. J. Mol. Med. 2017, 39, 1072–1082.
- Xia, E.; Zhai, L.; Huang, Z.; Liang, H.; Yang, H.; Song, G.; Li, W.; Tang, H. Optimization and identification of antioxidant peptide from underutilized Dunaliella salina protein: Extraction, gastrointestinal digestion, and fractionation. BioMed Res. Int. 2019, 2019, 6424651.
- Matsuo, H.; Mokudai, T.; Higo, M.; Nonaka, K.; Nagano, Y.; Nagahama, T.; Niwano, Y.; Takahashi, Y.; Omura, S.; Nakashima, T. Cipralphelin, a new anti-oxidative N-cinnamoyl tripeptide produced by the deep sea-derived fungal strain Penicillium brevicompactum FKJ-0123. J. Antibiot. 2019, 72, 775–778.
- Tripathi, V.C.; Horam, S.; Singh, A.; Lata, M.; Reddy, T.J.; Arockiaraj, J.; Pasupuleti, M. The discovery of antioxidants in marine microorganisms and their protective effects on the hepatic cells from chemical-induced oxidative stress. Free Rad. Res. 2020, 54, 150–161.
- Zhang, S.; Han, L.; Shi, Y.; Li, X.; Zhang, X.; Hou, H.; Lin, H.; Liu, K. Two Novel multi-functional peptides from meat and visceral mass of marine snail neptunea arthritica cumingii and their activities. Mar. Drugs 2018, 16, 473.
- Yang, X.; Qiu, Y.; Zhao, Y.; Chi, C.; Wang, B. Purification and characterization of antioxidant peptides derived from protein hydrolysate of the marine bivalve mollusk Tergillarca granosa. Mar. Drugs 2019, 17, 251.
- Byun, H.-G.; Lee, J.K.; Park, H.G.; Jeon, J.-K.; Kim, S.-K. Antioxidant peptides isolated from the marine rotifer, Brachionus rotundiformis. Process Biochem. 2009, 44, 842–846.
- Zhao, G.; Yang, X.; Wang, Y.; Zhao, Y.; Chi, C.; Wang, B. Antioxidant peptides from the protein hydrolysate of Spanish Mackerel (Scomberomorous niphonius) muscle by gastrointestinal digestion and their activities. Mar. Drugs 2019, 17, 531.
- Zhang, J.; Zhao, Y.; Wang, Y.; Chi, C.; Wang, B. Eight collagen peptides from hydrolysate fraction of Spanish Mackerel skins: Isolation, identification, and antioxidant activity evaluation. Mar. Drugs 2019, 17, 224.
- Pan, X.; Wang, Y.; Li, L.; Chi, C.; Wang, B. Four antioxidant peptides from protein hydrolysate of red stingray (Dasyatis akajei) cartilages: Isolation, identification, and activity evaluation. Mar. Drugs 2019, 17, 263.
- Zhang, L.; Zhao, G.; Zhao, Y.; Qiu, Y.; Chi, C.; Wang, B. Identification and active evaluation of antioxidant peptides from protein hydrolysates of skipjack tuna (Katsuwonus pelamis) head. Antioxidants 2019, 8, 318.
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; Alashi, M.A.; Aluko, R.E.; FitzGerald, R.J. Peptide identification in a salmon gelatin hydrolysate with antihypertensive, dipeptidyl peptidase IV inhibitory and antioxidant activities. Food Res. Int. 2017, 100, 112–120.
- Himaya, S.W.A.; Ngo, D.-H.; Ryu, B.; Kim, S.-K. An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress. Food Chem. 2012, 132, 1872–1882.
- Mendis, E.; Rajapakse, N.; Byun, H.G.; Kim, S.K. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005, 77, 2166–2178.
- Nazeer, R.A.; Kumar, N.S.S. Purification and identification of antioxidant peptide from black pomfret, Parastromateus niger (Bloch, 1975) viscera protein hydrolysate. Food Sci. Biotechnol. 2011, 20, 1087–1094.
- Abdelhedi, O.; Jridi, M.; Jemil, I.; Mora, L.; Toldrá, F.; Aristoy, M.-C.; Boualga, A.; Nasri, M.; Nasri, R. Combined biocatalytic conversion of smooth hound viscera: Protein hydrolysates elaboration and assessment of their an-tioxidant, anti-ACE and antibacterial activities. Food Res. Int. 2016, 86, 9–23.
- Kim, S.Y.; Je, J.Y.; Kim, S.K. Purification and characterization of antioxidant peptide from hoki (Johnius belenge-rii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007, 18, 31–38.
- Jun, S.Y.; Park, P.J.; Jung, W.K.; Kim, S.K. Purification and characterization of an antioxidative peptide from enzymatic hydrolysate of yellowfin sole (Limanda aspera) frame protein. Eur. Food Res. Technol. 2004, 219, 20–26.
- Je, J.-Y.; Qian, Z.-J.; Byun, H.-G.; Kim, S.-K. Purification and characterization of an antioxidant peptide obtaine-d from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007, 42, 840–846.
- Je, J.-Y.; Park, P.-J.; Kim, S.-K. Antioxidant activity of a peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res. Int. 2005, 38, 45–50.
- Li, W.; Lu, L.; Liu, B.; Qin, S. Effects of phycocyanin on pulmonary and gut microbiota in a radiation-induced pulmonary fibrosis model. Biomed. Pharmacother. 2020, 132, 110826.
- Han, K.; Jin, W.; Mao, Z.; Dong, S.; Zhang, Q.; Yang, Y.; Chen, B.; Wu, H.; Zeng, M. Microbiome and butyrate production are altered in the gut of rats fed a glycated fish protein diet. J. Func. Foods 2018, 47, 423–433.
- Wang, S.; Lv, Z.; Zhao, W.; Wang, L.; He, N. Collagen peptide from Walleye pollock skin attenuated obesity and modulated gut microbiota in high-fat diet-fed mice. J. Func. Foods 2020, 74, 04194.
- Mei, F.; Duan, Z.; Chen, M.; Lu, J.; Zhao, M.; Li, L.; Shen, X.; Xia, G.; Chen, S. Effect of a high-collagen peptide diet on the gut microbiota and short-chain fatty acid metabolism. J. Func. Foods 2020, 75, 104278.
- Durand, R.; Ouellette, A.; Houde, V.P.; Guénard, F.; Varin, T.V.; Marcotte, B.; Pilon, G.; Fraboulet, E.; Vohl, M.C.; Marette, A.; et al. Animal and cellular studies demonstrate some of the beneficial impacts of herring milt hydrolysates on obesity-induced glucose intolerance and inflammation. Nutrients 2020, 12, 3235.
- Wu, J.; Qiao, K.; Lu, H.; Chen, X.; Su, J.; Liu, Z. Abalone viscera protein hydrolysate modulation of gut microbiota in a mouse model of alcohol induced injury. J. Aquat. Food Prod. Technol. 2017, 26, 880–889.
- Hua, P.; Yu, Z.; Xiong, Y.; Liu, B.; Zhao, L. Regulatory efficacy of Spirulina platensis protease hydrolyzate on lipid metabolism and gut microbiota in high-fat diet-fed rats. Int. Mol. Sci. 2018, 19, 4023.
- Hua, P.; Xiong, Y.; Yu, Z.; Liu, B.; Zhao, L. Effect of Chlorella pyrenoidosa protein hydrolysate-calcium chelate on calcium absorption metabolism and gut microbiota composition in low-calcium diet-fed rats. Mar. Drugs 2019, 17, 6.
- Xiao, M.; Lin, L.; Chen, H.; Ge, X.; Huang, Y.; Zheng, Z.; Li, S.; Zeng, F. Anti-fatigue property of the oyster polypeptide fraction and its effect on gut microbiota in mice. Food Func. 2020, 11, 8659–8669.





