
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Talía Sainz | + 1229 word(s) | 1229 | 2021-12-17 03:25:57 | | | |
| 2 | Jessie Wu | Meta information modification | 1229 | 2021-12-20 02:35:20 | | |
Video Upload Options
The evolving field of microbiome research offers an excellent opportunity for biomarker identification, understanding drug metabolization disparities, and improving personalized medicine. However, the complexities of host–microbe ecological interactions hinder clinical transferability. Among other factors, the microbiome is deeply influenced by age and social determinants of health, including environmental factors such as diet and lifestyle conditions. In this entry, the bidirectionality of social and host–microorganism interactions in health will be discussed.
1. Introduction
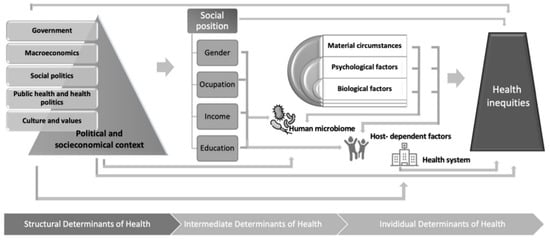
While the evidence supporting the interaction between the human host and the microbiome increases, the identification of microbiome-related biomarkers and the understanding of the role of microbiota in drug metabolization are also the focus of intense research. However, whether genetic or related to microbiota, validation of biomarkers among adults does not necessarily imply its usefulness in children, as gene expression and microbial colonization vary during childhood. It is known that the microbiome is established mainly during the first year of life, although fluctuations in the ecosystem occur over time together with lifetime changes [1]. Beyond age and genetic factors, the microbiome is deeply influenced by geographical, dietary, and lifestyle-related factors [2]. Studies suggest that these factors may be especially relevant in shaping the microbiome during childhood [3][1]. Social determinants of health have a direct impact on undoubtfully critical factors for health, such as malnutrition, access to treated water, or health care. In comparison, their effect on microbiota composition and how much these changes may contribute to health and disease may seem trivial and has not been well established.
2. Social Determinants of Health and the Microbiome in Children
References
- Mesa, M.D.; Loureiro, B.; Iglesia, I.; Gonzalez, S.F.; Olivé, E.L.; Algar, O.G.; Solana, M.J.; Perez, M.J.C.; Sainz, T.; Martinez, L.; et al. The Evolving Microbiome from Pregnancy to Early Infancy: A Comprehensive Review. Nutrients 2020, 12, 133.
- Gantenbein, K.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951.
- Taylor, M.; Allada, V.; Moritz, M.L.; Nowalk, A.J.; Sindhi, R.; Aneja, R.K.; Torok, K.; Morowitz, M.J.; Michaels, M.; Carcillo, J.A. Use of C-Reactive Protein and Ferritin Biomarkers in Daily Pediatric Practice. Pediatr. Rev. 2020, 41, 172–183.
- European Commission—Pharmaceutical Committee. Report on the Use of -Omic Technologies in the Development of Personalised Medicine. Available online: https://www.europarl.europa.eu/RegData/etudes/BRIE/2015/569009/EPRS_BRI(2015)569009_EN.pdf (accessed on 27 October 2015).
- World Health Organization. Social Determinants of Health . Available online: https://www.who.int/social_determinants/en/ (accessed on 30 January 2020).
- Narayan, A.; Raphael, J.; Rattler, T.; Bocchini, C. Social Determinants of Health—Screening in the Clinical Setting. Available online: https://www.texaschildrens.org/ (accessed on 13 October 2021).
- Braveman, P.; Egerter, S.; Williams, D.R. The Social Determinants of Health: Coming of Age. Annu. Rev. Public Health 2011, 32, 381–398.
- Jemal, A.; Thun, M.J.; Ward, E.E.; Henley, S.J.; Cokkinides, V.E.; Murray, T.E. Mortality from Leading Causes by Education and Race in the United States. Am.J. Prev. Med. 2008, 34, 1–8.e7.
- Sokol, R.; Austin, A.; Chandler, C.; Byrum, E.; Bousquette, J.; Lancaster, C.; Doss, G.; Dotson, A.; Urbaeva, V.; Singichetti, B.; et al. Screening Children for Social Determinants of Health: A Systematic Review. Pediatrics 2019, 144, e20191622.
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533.
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121.
- Moore, R.E.; Townsend, S.D. Temporal development of the infant gut microbiome. Open Biol. 2019, 9, 190128.
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; LeVan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191.
- Gaulke, C.A.; Sharpton, T.J. The influence of ethnicity and geography on human gut microbiome composition. Nat. Med. 2018, 24, 1495–1496.
- De Filippo, C.; Di Paola, M.; Ramazzotti, M.; Albanese, D.; Pieraccini, G.; Banci, E.; Miglietta, F.; Cavalieri, D.; Lionetti, P. Diet, environments, and gut microbiota. A preliminary investigation in children living in rural and urban burkina faso and italy. Front. Microbiol. 2017, 8, 1979.
- Ciabattini, A.; Olivieri, R.; Lazzeri, E.; Medaglini, D. Role of the Microbiota in the Modulation of Vaccine Immune Responses. Front. Microbiol. 2019, 10, 1305.
- Zimmermann, P.; Curtis, N. The influence of the intestinal microbiome on vaccine responses. Vaccine 2018, 36, 4433–4439.
- Loughman, A.; Ponsonby, A.-L.; O’Hely, M.; Symeonides, C.; Collier, F.; Tang, M.L.; Carlin, J.; Ranganathan, S.; Allen, K.; Pezic, A.; et al. Gut microbiota composition during infancy and subsequent behavioural outcomes. EBioMedicine 2020, 52, 102640.
- Mitchell, L.K.; Davies, P.S.W. Pre- and probiotics in the management of children with autism and gut issues: A review of the current evidence. Eur. J. Clin. Nutr. 2021, 1–9.
- Li, N.; Chen, H.; Cheng, Y.; Xu, F.; Ruan, G.; Ying, S.; Tang, W.; Chen, L.; Chen, M.; Lv, L.; et al. Fecal Microbiota Transplantation Relieves Gastrointestinal and Autism Symptoms by Improving the Gut Microbiota in an Open-Label Study. Front. Cell. Infect. Microbiol. 2021, 11, 948.
- Huey, S.L.; Jiang, L.; Fedarko, M.W.; McDonald, D.; Martino, C.; Ali, F.; Russell, D.G.; Udipi, S.A.; Thorat, A.; Thakker, V.; et al. Nutrition and the Gut Microbiota in 10- to 18-Month-Old Children Living in Urban Slums of Mumbai, India. mSphere 2020, 5, e00731-20.
- Serrano-Villar, S.; De Lagarde, M.; Castellanos, J.F.V.; Vallejo, A.; Bernadino, J.I.; Madrid, N.; Matarranz, M.; Díaz-Santiago, A.; Gutierrez, C.; Cabello, A.; et al. Effects of Immunonutrition in Advanced Human Immunodeficiency Virus Disease: A Randomized Placebo-controlled Clinical Trial (Promaltia Study). Clin. Infect. Dis. 2018, 68, 120–130.
- Khanna, S.; Raffals, L.E. The Microbiome in Crohn’s Disease. Gastroenterol. Clin. N. Am. 2017, 46, 481–492.
- Solomon, I.; Ilie, M.A.; Draghici, C.; Voiculescu, V.M.; Căruntu, C.; Boda, D.; Zurac, S. The impact of lifestyle factors on evolution of atopic dermatitis: An alternative approach (Review). Exp. Ther. Med. 2018, 17, 1078–1084.
- Burcham, Z.M.; Scientists, G.O.T.L.C.; Garneau, N.; Comstock, S.S.; Tucker, R.M.; Knight, R.; Metcalf, J.L. Patterns of Oral Microbiota Diversity in Adults and Children: A Crowdsourced Population Study. Sci. Rep. 2020, 10, 2133.
- Schwenger, E.M.; Tejani, A.M.; Loewen, P. Probiotics for preventing urinary tract infections in adults and children. Cochrane Database Syst. Rev. 2015, 12, CD008772.
- De Filippis, F.; Paparo, L.; Nocerino, R.; Della Gatta, G.; Carucci, L.; Russo, R.; Pasolli, E.; Ercolini, D.; Berni Canani, R. Specific gut microbiome signatures and the associated pro-inflammatory functions are linked to pediatric allergy and acquisition of immune tolerance. Nat. Commun. 2021, 12, 5958.
- Lopez-Santamarina, A.; Gonzalez, E.; Lamas, A.; Mondragon, A.; Regal, P.; Miranda, J. Probiotics as a Possible Strategy for the Prevention and Treatment of Allergies. A Narrative Review. Foods 2021, 10, 701.
- Harrison, C.J. Prevention of childhood leukaemia by lifestyle changes. Leukemia 2021, 35, 1265–1266.
- Masetti, R.; Muratore, E.; Leardini, D.; Zama, D.; Turroni, S.; Brigidi, P.; Esposito, S.; Pession, A. Gut microbiome in pediatric acute leukemia: From predisposition to cure. Blood Adv. 2021, 5, 4619–4629.
- Greaves, M.; Cazzaniga, V.; Ford, A. Can we prevent childhood Leukaemia? Leukemia 2021, 35, 1258–1264.




