Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Prakash Kumar Jha | + 1916 word(s) | 1916 | 2021-12-08 10:50:52 | | | |
| 2 | Yvaine Wei | Meta information modification | 1916 | 2021-12-17 04:57:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jha, P.K. Bioaccumulation of Fluoride and Its Microbially Assisted Remediation. Encyclopedia. Available online: https://encyclopedia.pub/entry/17239 (accessed on 07 February 2026).
Jha PK. Bioaccumulation of Fluoride and Its Microbially Assisted Remediation. Encyclopedia. Available at: https://encyclopedia.pub/entry/17239. Accessed February 07, 2026.
Jha, Prakash Kumar. "Bioaccumulation of Fluoride and Its Microbially Assisted Remediation" Encyclopedia, https://encyclopedia.pub/entry/17239 (accessed February 07, 2026).
Jha, P.K. (2021, December 17). Bioaccumulation of Fluoride and Its Microbially Assisted Remediation. In Encyclopedia. https://encyclopedia.pub/entry/17239
Jha, Prakash Kumar. "Bioaccumulation of Fluoride and Its Microbially Assisted Remediation." Encyclopedia. Web. 17 December, 2021.
Copy Citation
Fluoride is widely found in soil–water systems due to anthropogenic and geogenic activities that affect millions worldwide. Fluoride ingestion results in chronic and acute toxicity, including skeletal and dental fluorosis, neurological damage, and bone softening in humans.
fluoride
microbes
remediation
1. Introduction
Fluorine is a highly electronegative halogen [1] and essential component (13th) in the Earth’s crust with 625 mg kg−1 average concentration [2][3]. Fluoride exposure in the environment occurs through geogenic and anthropogenic sources. Fluoride in soil–water systems mainly occurs due to volcanic eruption, weathering, and leaching of rocks [4]. Thus, weathering and leaching of fluoride-bearing minerals are the primary sources of geogenic contamination and are typically associated with low calcium and high bicarbonate ions [5][6]. Churchill et al. [7] stated that coal also contains 295 mg L−1 of fluorine. Precipitation and air deposition are also geogenic sources of fluoride with concentrations in the range 0.00001 to 0.0004 mg L−1 or lower, as the fluoride analytical detection limit is 0.089 mg L−1 [8]. Anthropogenic activities are a potential source of fluoride ions in the soil–water systems. Anthropogenic sources include brick kilns, mining, pesticides and fertilizers, tiles, ceramics, and flux in steel and glass utilized in aluminum manufacturing [9][10][11][12]. In drinking water, the permissible limit established for fluoride by the World Health Organization (WHO) and Bureau of Indian Standards (BIS) is 1.5 mg L−1 [13][14][15]. According to the WHO report, more than 260 million individuals consume contaminated water of above 1 mg L−1 concentration [13].
Fluoride needs to be removed from the contaminated soil–water systems considering its harmful effects on humans, animals, and plants. Defluoridation of water can be accomplished using different techniques, such as coagulation, ion exchange, chemical precipitation, membrane separation, electrodialysis, electrolysis, and adsorption [9][11][16][17][18][19][20][21][22][23][24][25][26]. Currently, ion exchange resins are considered efficient materials for removing anionic and cationic pollutants from water. Although ion exchange is a fast and adjustable stoichiometric method of fluoride removal, it is expensive, generates a considerable amount of residue, and has complexity in resins; moreover, its interfering ion affects the removal efficiency [27][28]. In the adsorption process, adsorbents used for the fluoride removal are activated alumina [20][29], activated carbon prepared from Moringa indica bark [30], laterite [31], and waste residue [32]. However, these methods have limitations such as high energy consumption, high cost, production of secondary contaminants, and inefficiency in removing pollutants from wastewater [33]. Microorganisms are an alternative approach for fluoride removal that has recently gained prominence in toxic element removal. Microorganisms can effectively remediate heavy metals and also reduce and oxidize transition metals. Microbes can efficiently remove fluoride because of their bacterial cell wall composition, including amines, carboxylates, and phosphate [34][35][36]. Many microorganisms have been used to eliminate fluoride ions from wastewater, such as bacterium Acinetobacter sp. (GU566361) [33], bacterium Providencia vermicola (KX926492) [37], cyanobacteria [38], and Aspergillus niger [39]. Another advantage of using microbes for fluoride removal is its simple operation method, cost-effectiveness, low energy requirement, and minimal generation of secondary pollutants [37].
2. Sources and Geochemistry of Fluoride in Environmental Compartments
Natural sources contribute to a massive percentage of fluoride in soil, water, and plant systems (e.g., forage, grasses, and grains), which affects the occurrence and severity of fluorosis. Fluoride-enriched minerals in the subsurface zone cause high concentrations in natural water resources through geogenic contamination, as fluoride ions are being released from weak structural zones into soils and groundwater [40]. Rocks enriched with fluoride minerals are extensive fluoride reservoirs, as groundwater runs via fractures or pores of rocks or consolidated materials, such as granitic rocks; therefore, the availability of fluorine in the Earth’s crust supports emergence in groundwater [41]. Minerals containing fluoride, such as hornblende, biotite, and muscovite, have been discovered near volcanic rocks and could discharge fluoride into the groundwater [42].
In groundwater, fluoride contaminations are mainly from fluoride-bearing mineral rocks via dissolution, ion exchange, and sorbent surface desorption, including anthropogenic pollution. Four key processes that control hydrochemistry of groundwater resources are nitrate oxidation of organic carbon, silicate mineral weathering, soluble salt, and sulfate mineral dissolution in aquifers, in which fluoride enrichment is directly linked with weathering of silicate minerals [43]. The primary source of fluoride contamination in the groundwater is the dissolution of minerals such as fluorite and biotite in laterite sheeted basalt and granite gneiss. Consequently, the depth of the groundwater level also influences the fluoride contents [44]. For example, high fluoride concentrations in groundwater from deep aquifers and geothermal springs have been attributed to geothermal temperature as one of the driving mechanisms [45]. High fluoride concentrations have been reported in sub-Saharan Africa and East Africa due to volcanic activity. Volcanic ash is a natural source, has a high fluoride concentration, and is easily soluble in water [45].
Human activities (i.e., domestic waste, pesticide, and fertilizer use in agriculture) and industries pollute the environment with significant fluoride inputs to the subsurface aquatic system [46]. Agricultural fertilizers and coal burning are two major anthropogenic contributors to fluoride [47][48]. Phosphate fertilizers influence the fluoride level in irrigated lands [49]. At the same time, coal is being utilized for combustion in several industries and brick kilns. Inappropriate fly ash disposal on the surface of the ground leads to high fluoride concentration in the groundwater. The dispersion of particulate fluoride from the aerial emission reaches the surface and, after rains, percolates due to precipitation, reaching the groundwater zone [50][51].
3. Toxicity of Fluoride to Human and Animal Health
The influence of fluoride on mineralized tissues prevents tooth decay and enables enamel to be more resistant to acid attacks [52][53]. However, excessive fluoride intake causes human health risks, including chronic and acute toxicity [54]. Acute toxicity includes diarrhea, abdominal pain, vomiting, dehydration, and excessive salivation, which occur rapidly if intake exceeds the limit. Fluoride ingestion of 35–70 mg kg−1 of body weight orally can cause rapid adverse effects [55]. Long-term fluoride ingestion, even in small amounts of fluoride, can cause chronic toxicity. Prolonged fluoride consumption above 8 mg L−1 causes skeletal fluorosis [56], arthritis, cancer, osteoporosis, infertility, thyroid disorder, Alzheimer’s disease, and brain damage [1][11][57][58][59]. The high availability of fluoride in bones makes them brittle and eventually develops into a severe health issue called skeletal fluorosis [54]. Fluoride is converted into hydrogen fluoride in acidic environments such as the stomach. The neutrally charged hydrogen fluoride molecule easily passes through the cell membrane to improve intracellular intake [60]. Fluoride toxicity causes oxidative stress, i.e., production of ROS (reactive oxygen species) and RON (reactive nitrogen species), and interrupts natural antioxidant defense mechanisms [61]. Islam and Patel [62] reported that excess fluoride ingestion could affect proteins, lipids, carbohydrates, and vitamins. It also affects various organs, including kidney, heart, liver, lungs, and gastrointestinal tract [63]. People suffering from kidney issues have a higher risk of fluoride accumulation, leading to death [64]. Studies have reported that excessive fluoride use also affects the brain. Excess of fluoride impacts the peripheral nervous system function and structure and also causes a reduction in aerobic metabolism [65][66]. Excessive fluoride affects the kidney, including the urinary tract, and there may also be color change to red and itching near the axilla [67]. Fluoride can cause brittle bones and increase density and bone mass [67]. A report obtained from animal experiments shows that fluoride ingestion can affect the brain and antioxidant defense system [68]. The toxicity of fluoride to cattle has been reported to include a thyrotoxic effect on chronic fluorosis and disruption of the secretion of thyroxin hormones in mammalian cells [69][70][71]. Excessive fluoride affects more aquatic animals living in soft water than those living in hard water. This is due to the bioavailability of fluoride decreasing with an increase in water hardness; it can affect the growth of the organisms [67].
4. Factors Affecting Mobility and Bioavailability of Fluoride in Subsurface and Surface Systems
4.1. Groundwater
Major sources for fluoride contamination in groundwater include fluoride-bearing minerals in the host rocks and their interaction with groundwater through chemical processes such as breakdown, dissolution, and dissociation. Evaporation, chemical weathering, and ion exchange are the key geochemical regulating factors of fluoride enrichment in groundwater [72]. Fluoride enrichment in groundwater is most commonly caused by the dissolving of the fluoride-rich minerals such as amphiboles and micas found in aquifers, which are primarily granitic-rich rocks. Longer residence time results in substantial water–rock interactions for fluoride mineral resolution [73]. In shallow groundwater, soluble salts are gradually reduced, pH is increased, and the dissolution fluorite equilibrium is observed in areas with high fluoride concentration [74]. The predominant hydrogeochemical mechanisms in groundwater are silicate weathering and ion exchange. Therefore, weathering and cation exchange influence the major groundwater chemistry, significantly affecting fluoride enrichment; an essential natural activity is controlling groundwater chemistry [75].
4.2. Soil
Fluoride in soil was found to be a mobile element. Furthermore, relative mobility suggested that soils played a more significant role in releasing fluoride into groundwater than rocks. Fluoride solubility in the soil is profoundly affected by soil pH, texture, organic matter, and concentration of other ions [76][77][78][79][80]. In soil, fluoride concentrations vary from 100 to 400 mg L−1 [12]; fluoride in soil is primarily combined with clay fraction of the soil colloids, and its mobility depends on the type of sorbents, pH, soil salinity, and sorption capacity [81][12][82]. For example, acidic soils can increase the bioavailability of fluoride; however, solubility decreases at pH in the range of 6–6.5 [76][77][83]. Fluoride generally combines with aluminum or calcium in the soil, so clay soil or silts have a higher fluoride concentration than sandy soil [84]. Fluoride mobility is affected at the high salinity; i.e., other ions in high concentration compete with fluoride for sorption sites [12]. Moreover, evapotranspiration can also regulate soil fluoride concentration and salinity [85]. Fluoride is mainly immobile in soil because it is not readily soluble and exchangeable, but soluble fluoride is vital to plant and animal growth [86][87].
5. Fluoride Uptake and Bioaccumulation in Plants and Foods
Fluoride is a common phytotoxic contaminant for plants [88]. Plant species absorb fluoride from water and soil via roots and air through leaves [88][89], accumulate fluoride in different parts of the plant, and acquire fluoride at a higher level than in the environment [90][91]. Fluoride accumulation in plants depends on various factors such as fluoride concentration in soil, plant species, and soil properties [84]. Fluoride accumulation in soil surrounds roots and disturbs the biochemical, morphological, and physiological behavior of plants. Plants grown in uncontaminated soils have an average fluoride concentration of less than 10 mg kg−1 [92]. Besides this, fluoride toxicity in plants depends on the level, frequency, and duration of exposure and the genotype of plants [1][93]. The presence of other anions and changes in pH also influences the fluoride accumulation in soil [57]. The growth and productivity of crop species and other plants are also affected detrimentally because of fluoride exposure, even at lower concentrations [94].
Figure 1 shows how plants uptake fluoride from contaminated soil and air, translocate it and accumulate it in cell walls. Plants are vulnerable to fluoride accumulation, and their growth and development process can be affected negatively even with a lower level of fluoride deposition [94]. However, plants such as Zea mays and Lupinus luteus were found fluoride-tolerant because of their ability of protein synthesis and self-protection against protein degradation [95].
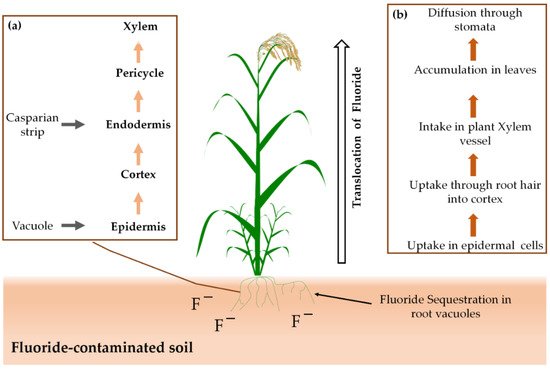
Figure 1. Fluoride uptake and accumulation in plants: (a) uptake mechanism in roots and (b) overall translocation from roots to shoots.
6. Microbial Remediation Techniques for Fluoride Removal
Microbial techniques are based upon using native or genetically modified microorganisms to remove fluoride ions from contaminated areas for environmental protection [36]. Different types of bacteria and other microorganisms rarely exhibited any toxic effect while exposed to high fluoride concentrations [96]. The antimicrobial effect of fluoride occurs by direct inhibition of enzymes by fluoride ion or hydrogen fluoride through the formation of phosphate analogs, such as alumino-fluoride or beryllium fluoride, that affect phosphate group transferring enzymes by inhibiting the adenosine triphosphate (ATP) synthesis. Microorganisms can perform various processes, such as mineralization, metal uptake, accumulation, sorption, enzymatic oxidation and reduction, extracellular precipitation, and efflux of xenobiotics using ionospheres to overcome fluoride toxicity [35][97].
References
- Singh, G.; Kumari, B.; Sinam, G.; Kumar, N.; Mallick, S. Fluoride distribution and contamination in the water, soil and plants continuum and its remedial technologies, an Indian perspective—A review. Environ. Pollut. 2018, 239, 95–108.
- Camargo, J.A.; Alonso, Á. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849.
- Selinus, O.; Alloway, B.; Centeno, J.A.; Finkelman, R.B.; Fuge, R.; Lindh, U.; Smedley, P. Essentials of Medical Geology; Springer: Berlin/Heidelberg, Germany, 2016.
- Yadav, M.; Singh, G.; Jadeja, R. Fluoride Contamination in Groundwater, Impacts, and Their Potential Remediation Techniques. Groundw. Geochem. Pollut. Remediat. Methods 2021, 22–41.
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water; Department of the Interior, US Geological Survey: Washington, DC, USA, 1985; Volume 2254.
- Handa, B. Geochemistry and genesis of Fluoride-Containing ground waters in india. Groundwater 1975, 13, 275–281.
- Churchill, H.; Rowley, R.; Martin, L. Flourine Content of Certain Vegetation in Western Pennsylvania Area. Anal. Chem. 1948, 20, 69–71.
- Gupta, S.; Deshpande, R.; Agarwal, M.; Raval, B. Origin of high fluoride in groundwater in the North Gujarat-Cambay region, India. Hydrogeol. J. 2005, 13, 596–605.
- Ali, S.; Thakur, S.K.; Sarkar, A.; Shekhar, S. Worldwide contamination of water by fluoride. Environ. Chem. Lett. 2016, 14, 291–315.
- Datta, P.; Deb, D.; Tyagi, S. Stable isotope (18O) investigations on the processes controlling fluoride contamination of groundwater. J. Contam. Hydrol. 1996, 24, 85–96.
- Bibi, S.; Kamran, M.A.; Sultana, J.; Farooqi, A. Occurrence and methods to remove arsenic and fluoride contamination in water. Environ. Chem. Lett. 2017, 15, 125–149.
- Hong, B.-D.; Joo, R.-N.; Lee, K.-S.; Lee, D.-S.; Rhie, J.-H.; Min, S.-W.; Song, S.-G.; Chung, D.-Y. Fluoride in soil and plant. Korean J. Agric. Sci. 2016, 43, 522–536.
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004; Volume 1.
- BIS. Drinking Water-Specification B.S.10500; Bureau of Indian Standard: New Delhi, India, 2012. Available online: http://cgwb.gov.in/Documents/WQ-standards.pdf (accessed on 20 October 2021).
- WHO. Surveillance and control of community supplies. In Surveillance and Control of Community Supplies; WHO: Geneva, Switzerland, 1997; p. 238.
- Roy, S.; Dass, G. Fluoride contamination in drinking water—A review. Resour. Environ. 2013, 3, 53–58.
- Bhatnagar, A.; Kumar, E.; Sillanpää, M. Fluoride removal from water by adsorption—A review. Chem. Eng. J. 2011, 171, 811–840.
- Jha, P.K.; Tripathi, P. Arsenic and fluoride contamination in groundwater: A review of global scenarios with special reference to India. Groundw. Sustain. Dev. 2021, 13, 100576.
- Maity, J.P.; Hsu, C.-M.; Lin, T.-J.; Lee, W.-C.; Bhattacharya, P.; Bundschuh, J.; Chen, C.-Y. Removal of fluoride from water through bacterial-surfactin mediated novel hydroxyapatite nanoparticle and its efficiency assessment: Adsorption isotherm, adsorption kinetic and adsorption thermodynamics. Environ. Nanotechnol. Monit. Manag. 2018, 9, 18–28.
- Kumar, R.; Sharma, P.; Aman, A.K.; Singh, R.K. Equilibrium sorption of fluoride on the activated alumina in aqueous solution. Desalination Water Treat. 2020, 197, 224–236.
- Singh, G.; Kumar, B.; Sen, P.; Majumdar, J. Removal of fluoride from spent pot liner leachate using ion exchange. Water Environ. Res. 1999, 71, 36–42.
- Saha, S. Treatment of aqueous effluent for fluoride removal. Water Res. 1993, 27, 1347–1350.
- Reardon, E.J.; Wang, Y. A limestone reactor for fluoride removal from wastewaters. Environ. Sci. Technol. 2000, 34, 3247–3253.
- Amor, Z.; Bariou, B.; Mameri, N.; Taky, M.; Nicolas, S.; Elmidaoui, A. Fluoride removal from brackish water by electrodialysis. Desalination 2001, 133, 215–223.
- Mameri, N.; Lounici, H.; Belhocine, D.; Grib, H.; Piron, D.; Yahiat, Y. Defluoridation of Sahara water by small plant electrocoagulation using bipolar aluminium electrodes. Sep. Purif. Technol. 2001, 24, 113–119.
- Hichour, M.; Persin, F.; Sandeaux, J.; Gavach, C. Fluoride removal from waters by Donnan dialysis. Sep. Purif. Technol. 1999, 18, 1–11.
- Barathi, M.; Kumar, A.S.K.; Rajesh, N. Impact of fluoride in potable water—An outlook on the existing defluoridation strategies and the road ahead. Coord. Chem. Rev. 2019, 387, 121–128.
- Jayashreea, D.E.; Poojaa, G.; Kumara, P.S.; Prasannamedhaa, G. A review on fluoride: Treatment strategies and scope for further research. Desalination Water Treat. 2020, 200, 167–186.
- Tripathy, S.S.; Bersillon, J.-L.; Gopal, K. Removal of fluoride from drinking water by adsorption onto alum-impregnated activated alumina. Sep. Purif. Technol. 2006, 50, 310–317.
- Karthikeyan, G.; Ilango, S.S. Fluoride sorption using Morringa Indica-based activated carbon. J. Environ. Health Sci. Eng. 2007, 4, 21–28.
- Sarkar, M.; Banerjee, A.; Pramanick, P.P.; Sarkar, A.R. Design and operation of fixed bed laterite column for the removal of fluoride from water. Chem. Eng. J. 2007, 131, 329–335.
- Nigussie, W.; Zewge, F.; Chandravanshi, B. Removal of excess fluoride from water using waste residue from alum manufacturing process. J. Hazard. Mater. 2007, 147, 954–963.
- Shanker, A.S.; Srinivasulu, D.; Pindi, P.K. A study on bioremediation of fluoride-contaminated water via a novel bacterium Acinetobacter sp. (GU566361) isolated from potable water. Results Chem. 2020, 2, 100070.
- Kleinübing, S.; Da Silva, E.; Da Silva, M.; Guibal, E. Equilibrium of Cu (II) and Ni (II) biosorption by marine alga Sargassum filipendula in a dynamic system: Competitiveness and selectivity. Bioresour. Technol. 2011, 102, 4610–4617.
- Chouhan, S.; Tuteja, U.; Flora, S. Isolation, identification and characterization of fluoride resistant bacteria: Possible role in bioremediation. Appl. Biochem. Microbiol. 2012, 48, 43–50.
- Juwarkar, A.A.; Yadav, S.K. Bioaccumulation and Biotransformation of Heavy Metals. In Bioremediation Technology: Recent Advances; Fulekar, M.H., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 266–284.
- Mukherjee, S.; Sahu, P.; Halder, G. Microbial remediation of fluoride-contaminated water via a novel bacterium Providencia vermicola (KX926492). J. Environ. Manag. 2017, 204, 413–423.
- Biswas, G.; Thakurta, S.G.; Chakrabarty, J.; Adhikari, K.; Dutta, S. Evaluation of fluoride bioremediation and production of biomolecules by living cyanobacteria under fluoride stress condition. Ecotoxicol. Environ. Saf. 2018, 148, 26–36.
- Annadurai, S.T.; Arivalagan, P.; Sundaram, R.; Mariappan, R.; Munusamy, A.P. Batch and column approach on biosorption of fluoride from aqueous medium using live, dead and various pretreated Aspergillus niger (FS18) biomass. Surf. Interfaces 2019, 15, 60–69.
- Bera, B.; Ghosh, A. Fluoride dynamics in hydrogeological diversity and Fluoride Contamination Index mapping: A correlation study of North Singbhum Craton, India. Arab. J. Geosci. 2019, 12, 1–15.
- Hanse, A.; Chabukdhara, M.; Baruah, S.G.; Boruah, H.; Gupta, S.K. Fluoride contamination in groundwater and associated health risks in Karbi Anglong District, Assam, Northeast India. Environ. Monit. Assess. 2019, 191, 1–17.
- Amiri, V.; Berndtsson, R. Fluoride occurrence and human health risk from groundwater use at the west coast of Urmia Lake, Iran. Arab. J. Geosci. 2020, 13, 1–23.
- Yidana, S.M.; Ophori, D.; Banoeng-Yakubo, B.; Samed, A.A. A factor model to explain the hydrochemistry and causes of fluoride enrichment in groundwater from the middle Voltaian sedimentary aquifers in the northern region, Ghana. ARPN J. Eng. Appl. Sci. 2012, 7, 50–68.
- Hossain, M.; Patra, P.K. Hydrogeochemical characterisation and health hazards of fluoride enriched groundwater in diverse aquifer types. Environ. Pollut. 2020, 258, 113646.
- Onipe, T.; Edokpayi, J.N.; Odiyo, J.O. A review on the potential sources and health implications of fluoride in groundwater of Sub-Saharan Africa. J. Environ. Sci. Health Part A 2020, 55, 1078–1093.
- Luo, W.; Gao, X.; Zhang, X. Geochemical processes controlling the groundwater chemistry and fluoride contamination in the Yuncheng Basin, China—An area with complex hydrogeochemical conditions. PLoS ONE 2018, 13, e0199082.
- Mikkonen, H.G.; van de Graaff, R.; Mikkonen, A.T.; Clarke, B.O.; Dasika, R.; Wallis, C.J.; Reichman, S.M. Environmental and anthropogenic influences on ambient background concentrations of fluoride in soil. Environ. Pollut. 2018, 242, 1838–1849.
- Kaur, L.; Rishi, M.S.; Siddiqui, A.U. Deterministic and probabilistic health risk assessment techniques to evaluate non-carcinogenic human health risk (NHHR) due to fluoride and nitrate in groundwater of Panipat, Haryana, India. Environ. Pollut. 2020, 259, 113711.
- Yu, Y.-Q.; Cui, S.-F.; Fan, R.-J.; Fu, Y.-Z.; Liao, Y.-L.; Yang, J.-Y. Distribution and superposed health risk assessment of fluorine co-effect in phosphorous chemical industrial and agricultural sources. Environ. Pollut. 2020, 262, 114249.
- Brindha, K.; Elango, L. Fluoride in groundwater: Causes, implications and mitigation measures. Fluoride Prop. Appl. Environ. Manag. 2011, 1, 111–136.
- Masood, N.; Hudson-Edwards, K.A.; Farooqi, A. Groundwater nitrate and fluoride profiles, sources and health risk assessment in the coal mining areas of Salt Range, Punjab Pakistan. Environ. Geochem. Health 2021, 1–14.
- Maheshwari, R. Fluoride in drinking water and its removal. J. Hazard. Mater. 2006, 137, 456–463.
- Buzalaf, M.A.R.; Whitford, G.M. Fluoride metabolism. In Fluoride and the Oral Environment; Buzalaf, M.A.R., Ed.; Monogr Oral Science Basel, Karger: Basel, Switzerland, 2011; Volume 22, pp. 20–36.
- Dhar, V.; Bhatnagar, M. Physiology and toxicity of fluoride. Indian J. Dent. Res. 2009, 20, 350.
- Mellberg, J.R.; Ripa, L.W.; Leske, G.S. Fluorido in preventive dentistryTheory and clinical applications. Kerman Univ. Med. Sci. 1983, 1, 290.
- Yadav, A.K.; Kaushik, C.P.; Haritash, A.K.; Singh, B.; Raghuvanshi, S.P.; Kansal, A. Determination of exposure and probable ingestion of fluoride through tea, toothpaste, tobacco and pan masala. J. Hazard. Mater. 2007, 142, 77–80.
- Ruan, J.; Ma, L.; Shi, Y.; Han, W. The impact of pH and calcium on the uptake of fluoride by tea plants (Camellia sinensis L.). Ann. Bot. 2004, 93, 97–105.
- Whitford, G.M. The Metabolism and Toxicity of Fluoride; Karger Publishers: Basel, Switzerland, 1996.
- Harrison, P.T. Fluoride in water: A UK perspective. J. Fluor. Chem. 2005, 126, 1448–1456.
- Barbier, O.; Arreola-Mendoza, L.; Del Razo, L.M. Molecular mechanisms of fluoride toxicity. Chem. Biol. Interact. 2010, 188, 319–333.
- Maritim, A.; Sanders, R.; Watkins, J., 3rd. Oxidative stress and diabetic complications: A review. J. Biochem. Mol. Toxic 2003, 17, 24–38.
- Islam, M.; Patel, R. Thermal activation of basic oxygen furnace slag and evaluation of its fluoride removal efficiency. Chem. Eng. J. 2011, 169, 68–77.
- Perumal, E.; Paul, V.; Govindarajan, V.; Panneerselvam, L. A brief review on experimental fluorosis. Toxicol. Lett. 2013, 223, 236–251.
- Xiong, X.; Liu, J.; He, W.; Xia, T.; He, P.; Chen, X.; Yang, K.; Wang, A. Dose–effect relationship between drinking water fluoride levels and damage to liver and kidney functions in children. Environ. Res. 2007, 103, 112–116.
- Bhatnagar, M.; Rao, P.; Saxena, A.; Bhatnagar, R.; Meena, P.; Barbar, S.; Chouhan, A.; Vimal, S. Biochemical changes in brain and other tissues of young adult female mice from fluoride in their drinking water. Fluoride 2006, 39, 280–284.
- Vani, M.L.; Reddy, K.P. Effects of fluoride accumulation on some enzymes of brain and gastrocnemius muscle of mice. Fluoride 2000, 33, 17–26.
- Ghosh, A.; Mukherjee, K.; Ghosh, S.K.; Saha, B. Sources and toxicity of fluoride in the environment. Res. Chem. Intermed. 2013, 39, 2881–2915.
- Kabir, H.; Gupta, A.K.; Tripathy, S. Fluoride and human health: Systematic appraisal of sources, exposures, metabolism, and toxicity. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1116–1193.
- Bouaziz, H.; Ammar, E.; Ghorbel, H.; Ketata, S.; Jamoussi, K.; Ayadi, F.; Guermazi, F.; Zeghal, N. Effect of fluoride ingested by lactating mice on the thyroid function and bone maturation of their suckling pups. Fluoride 2004, 37, 133–142.
- Cinar, A.; Selcuk, M. Effects of chronic fluorosis on thyroxine, triiodothyronine, and protein-bound iodine in cows. Fluoride 2005, 38, 65–68.
- Rahman, M.M.A.; Fetouh, F.A. Effect of sodium fluoride on the thyroid follicular cells and the amelioration by calcium supplementation in Albino rats: A light and an electron microscopic study. J. Am. Sci. 2013, 9, 107–114.
- Dehbandi, R.; Moore, F.; Keshavarzi, B. Geochemical sources, hydrogeochemical behavior, and health risk assessment of fluoride in an endemic fluorosis area, central Iran. Chemosphere 2018, 193, 763–776.
- Jia, H.; Qian, H.; Qu, W.; Zheng, L.; Feng, W.; Ren, W. Fluoride occurrence and human health risk in drinking water wells from southern edge of Chinese Loess Plateau. Int. J. Environ. Res. Public Health 2019, 16, 1683.
- Shi, M.; Gao, Z.; Feng, J.; Zhang, H.; Cui, Y.; Fang, S.; Liu, J. Characteristics and effects of fluorine release from shallow high-fluoride soils. Environ. Earth Sci. 2019, 78, 1–10.
- Adimalla, N.; Li, P. Occurrence, health risks, and geochemical mechanisms of fluoride and nitrate in groundwater of the rock-dominant semi-arid region, Telangana State, India. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 81–103.
- Shu, W.; Zhang, Z.; Lan, C.; Wong, M.H. Fluoride and aluminium concentrations of tea plants and tea products from Sichuan Province, PR China. Chemosphere 2003, 52, 1475–1482.
- Viero, A.; Roisenberg, C.; Roisenberg, A.; Vigo, A. The origin of fluoride in the granitic aquifer of Porto Alegre, Southern Brazil. Environ. Geol. 2009, 56, 1707–1719.
- Wenzel, W.W.; Blum, W.E. Fluorine speciation and mobility in F-contaminated soils. Soil Sci. 1992, 153, 357–364.
- Cronin, S.; Manoharan, V.; Hedley, M.; Loganathan, P. Fluoride: A review of its fate, bioavailability, and risks of fluorosis in grazed-pasture systems in New Zealand. N. Z. J. Agric. Res. 2000, 43, 295–321.
- Yi, X.; Qiao, S.; Ma, L.; Wang, J.; Ruan, J. Soil fluoride fractions and their bioavailability to tea plants (Camellia sinensis L.). Environ. Geochem. Health 2017, 39, 1005–1016.
- Pickering, W. The mobility of soluble fluoride in soils. Environ. Pollut. Ser. B Chem. Phys. 1985, 9, 281–308.
- Fuhong, R.; Shuqin, J. Distribution and formation of high-fluorine groundwater in China. Environ. Geol. Water Sci. 1988, 12, 3–10.
- Loganathan, P.; Gray, C.; Hedley, M.; Roberts, A. Total and soluble fluorine concentrations in relation to properties of soils in New Zealand. Eur. J. Soil Sci. 2006, 57, 411–421.
- Tylenda, C.A. Toxicological Profile for Fluorides, Hydrogen Fluoride, and Fluorine; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2003.
- Edmunds, W.; Smedley, P. Fluoride in Natural Waters; Selinus, O., Alloway, B., Centeno, J.A., Finkelman, R.B., Fuge, R., Lundh, U., Smedley, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2005.
- Gilpin, L.; Johnson, A.H. Fluorine in agricultural soils of southeastern Pennsylvania. Soil Sci. Soc. Am. J. 1980, 44, 255–258.
- Davison, A. Uptake, transport and accumulation of soil and airborne fluorides by vegetation. In Fluorides: Effects on Vegetation, Animals and Humans; Shupe, J., Peterson, H., Leone, N., Eds.; Paragon Press: Salt Lake City, UT, USA, 1983; pp. 61–82.
- Zhang, L.; Li, Q.; Ma, L.; Ruan, J. Characterization of fluoride uptake by roots of tea plants (Camellia sinensis (L.) O. Kuntze). Plant Soil 2013, 366, 659–669.
- Gadi, B.; Kumar, R.; Goswami, B.; Rankawat, R.; Rao, S.R. Recent developments in understanding fluoride accumulation, toxicity, and tolerance mechanisms in plants: An overview. J. Soil Sci. Plant Nutr. 2021, 21, 209–228.
- Anshumali, B. Fluoride in agricultural soil: A review on its sources and toxicity to plants. Glob. Sustain. Transit. Impacts Innov. 2014, 3, 29–37.
- Smolik, B.; Telesiński, A.; Szymczak, J.; Zakrzewska, H. Assessing of humus usefulness in limiting of soluble fluoride content in soil. Environ. Prot. Nat. Resour. 2011, 49, 202–208.
- Davison, A.; Takmaz-Nisancioglu, S.; Bailey, I. The Dynamics of Fluoride Accumulation by Vegetation Fluoride Toxicity; International Society for Fluoride Research: New Delhi, India, 1985; Volume 46.
- Sharma, R.; Kaur, R. Insights into fluoride-induced oxidative stress and antioxidant defences in plants. Acta Physiol. Plant. 2018, 40, 1–14.
- Sahariya, A.; Bharadwaj, C.; Emmanuel, I.; Alam, A. Fluoride toxicity in soil and plants: An overview. Asian J. Adv. Res. 2021, 26–34.
- Banerjee, A.; Roychoudhury, A. Fluorine: A biohazardous agent for plants and phytoremediation strategies for its removal from the environment. Biol Plant 2019, 63, 104–112.
- Baunthiyal, M.; Ranghar, S. Accumulation of fluoride by plants: Potential for phytoremediation. Clean Soil Air Water 2015, 43, 127–132.
- Kalyani, G.; Rao, G.B.; Vijaya, B.; Kumar, Y.P. Biosorption isotherms of fluoride from aqueous solution on Ulva fasciata sp.–A waste material. Int. J. Appl. Environ. Sci. 2009, 4, 173–182.
More
Information
Subjects:
Water Resources
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
17 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




