Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Water Resources
Fluoride is widely found in soil–water systems due to anthropogenic and geogenic activities that affect millions worldwide. Fluoride ingestion results in chronic and acute toxicity, including skeletal and dental fluorosis, neurological damage, and bone softening in humans.
- fluoride
- microbes
- remediation
1. Introduction
Fluorine is a highly electronegative halogen [1] and essential component (13th) in the Earth’s crust with 625 mg kg−1 average concentration [2,3]. Fluoride exposure in the environment occurs through geogenic and anthropogenic sources. Fluoride in soil–water systems mainly occurs due to volcanic eruption, weathering, and leaching of rocks [6]. Thus, weathering and leaching of fluoride-bearing minerals are the primary sources of geogenic contamination and are typically associated with low calcium and high bicarbonate ions [4,7]. Churchill et al. [8] stated that coal also contains 295 mg L−1 of fluorine. Precipitation and air deposition are also geogenic sources of fluoride with concentrations in the range 0.00001 to 0.0004 mg L−1 or lower, as the fluoride analytical detection limit is 0.089 mg L−1 [9]. Anthropogenic activities are a potential source of fluoride ions in the soil–water systems. Anthropogenic sources include brick kilns, mining, pesticides and fertilizers, tiles, ceramics, and flux in steel and glass utilized in aluminum manufacturing [10,11,12,13]. In drinking water, the permissible limit established for fluoride by the World Health Organization (WHO) and Bureau of Indian Standards (BIS) is 1.5 mg L−1 [14,15,16]. According to the WHO report, more than 260 million individuals consume contaminated water of above 1 mg L−1 concentration [14].
Fluoride needs to be removed from the contaminated soil–water systems considering its harmful effects on humans, animals, and plants. Defluoridation of water can be accomplished using different techniques, such as coagulation, ion exchange, chemical precipitation, membrane separation, electrodialysis, electrolysis, and adsorption [10,12,83,84,85,86,87,88,89,90,91,92,93]. Currently, ion exchange resins are considered efficient materials for removing anionic and cationic pollutants from water. Although ion exchange is a fast and adjustable stoichiometric method of fluoride removal, it is expensive, generates a considerable amount of residue, and has complexity in resins; moreover, its interfering ion affects the removal efficiency [94,95]. In the adsorption process, adsorbents used for the fluoride removal are activated alumina [87,96], activated carbon prepared from Moringa indica bark [97], laterite [98], and waste residue [99]. However, these methods have limitations such as high energy consumption, high cost, production of secondary contaminants, and inefficiency in removing pollutants from wastewater [100]. Microorganisms are an alternative approach for fluoride removal that has recently gained prominence in toxic element removal. Microorganisms can effectively remediate heavy metals and also reduce and oxidize transition metals. Microbes can efficiently remove fluoride because of their bacterial cell wall composition, including amines, carboxylates, and phosphate [101,102,103]. Many microorganisms have been used to eliminate fluoride ions from wastewater, such as bacterium Acinetobacter sp. (GU566361) [100], bacterium Providencia vermicola (KX926492) [104], cyanobacteria [105], and Aspergillus niger [106]. Another advantage of using microbes for fluoride removal is its simple operation method, cost-effectiveness, low energy requirement, and minimal generation of secondary pollutants [104].
2. Sources and Geochemistry of Fluoride in Environmental Compartments
Natural sources contribute to a massive percentage of fluoride in soil, water, and plant systems (e.g., forage, grasses, and grains), which affects the occurrence and severity of fluorosis. Fluoride-enriched minerals in the subsurface zone cause high concentrations in natural water resources through geogenic contamination, as fluoride ions are being released from weak structural zones into soils and groundwater [107]. Rocks enriched with fluoride minerals are extensive fluoride reservoirs, as groundwater runs via fractures or pores of rocks or consolidated materials, such as granitic rocks; therefore, the availability of fluorine in the Earth’s crust supports emergence in groundwater [108]. Minerals containing fluoride, such as hornblende, biotite, and muscovite, have been discovered near volcanic rocks and could discharge fluoride into the groundwater [109].
In groundwater, fluoride contaminations are mainly from fluoride-bearing mineral rocks via dissolution, ion exchange, and sorbent surface desorption, including anthropogenic pollution. Four key processes that control hydrochemistry of groundwater resources are nitrate oxidation of organic carbon, silicate mineral weathering, soluble salt, and sulfate mineral dissolution in aquifers, in which fluoride enrichment is directly linked with weathering of silicate minerals [110]. The primary source of fluoride contamination in the groundwater is the dissolution of minerals such as fluorite and biotite in laterite sheeted basalt and granite gneiss. Consequently, the depth of the groundwater level also influences the fluoride contents [111]. For example, high fluoride concentrations in groundwater from deep aquifers and geothermal springs have been attributed to geothermal temperature as one of the driving mechanisms [112]. High fluoride concentrations have been reported in sub-Saharan Africa and East Africa due to volcanic activity. Volcanic ash is a natural source, has a high fluoride concentration, and is easily soluble in water [112].
Human activities (i.e., domestic waste, pesticide, and fertilizer use in agriculture) and industries pollute the environment with significant fluoride inputs to the subsurface aquatic system [113]. Agricultural fertilizers and coal burning are two major anthropogenic contributors to fluoride [114,115]. Phosphate fertilizers influence the fluoride level in irrigated lands [116]. At the same time, coal is being utilized for combustion in several industries and brick kilns. Inappropriate fly ash disposal on the surface of the ground leads to high fluoride concentration in the groundwater. The dispersion of particulate fluoride from the aerial emission reaches the surface and, after rains, percolates due to precipitation, reaching the groundwater zone [117,118].
3. Toxicity of Fluoride to Human and Animal Health
The influence of fluoride on mineralized tissues prevents tooth decay and enables enamel to be more resistant to acid attacks [18,127]. However, excessive fluoride intake causes human health risks, including chronic and acute toxicity [128]. Acute toxicity includes diarrhea, abdominal pain, vomiting, dehydration, and excessive salivation, which occur rapidly if intake exceeds the limit. Fluoride ingestion of 35–70 mg kg−1 of body weight orally can cause rapid adverse effects [129]. Long-term fluoride ingestion, even in small amounts of fluoride, can cause chronic toxicity. Prolonged fluoride consumption above 8 mg L−1 causes skeletal fluorosis [130], arthritis, cancer, osteoporosis, infertility, thyroid disorder, Alzheimer’s disease, and brain damage [1,12,131,132,133]. The high availability of fluoride in bones makes them brittle and eventually develops into a severe health issue called skeletal fluorosis [128]. Fluoride is converted into hydrogen fluoride in acidic environments such as the stomach. The neutrally charged hydrogen fluoride molecule easily passes through the cell membrane to improve intracellular intake [134]. Fluoride toxicity causes oxidative stress, i.e., production of ROS (reactive oxygen species) and RON (reactive nitrogen species), and interrupts natural antioxidant defense mechanisms [135]. Islam and Patel [136] reported that excess fluoride ingestion could affect proteins, lipids, carbohydrates, and vitamins. It also affects various organs, including kidney, heart, liver, lungs, and gastrointestinal tract [137]. People suffering from kidney issues have a higher risk of fluoride accumulation, leading to death [138]. Studies have reported that excessive fluoride use also affects the brain. Excess of fluoride impacts the peripheral nervous system function and structure and also causes a reduction in aerobic metabolism [139,140]. Excessive fluoride affects the kidney, including the urinary tract, and there may also be color change to red and itching near the axilla [141]. Fluoride can cause brittle bones and increase density and bone mass [141]. A report obtained from animal experiments shows that fluoride ingestion can affect the brain and antioxidant defense system [142]. The toxicity of fluoride to cattle has been reported to include a thyrotoxic effect on chronic fluorosis and disruption of the secretion of thyroxin hormones in mammalian cells [143,144,145]. Excessive fluoride affects more aquatic animals living in soft water than those living in hard water. This is due to the bioavailability of fluoride decreasing with an increase in water hardness; it can affect the growth of the organisms [141].
4. Factors Affecting Mobility and Bioavailability of Fluoride in Subsurface and Surface Systems
4.1. Groundwater
Major sources for fluoride contamination in groundwater include fluoride-bearing minerals in the host rocks and their interaction with groundwater through chemical processes such as breakdown, dissolution, and dissociation. Evaporation, chemical weathering, and ion exchange are the key geochemical regulating factors of fluoride enrichment in groundwater [146]. Fluoride enrichment in groundwater is most commonly caused by the dissolving of the fluoride-rich minerals such as amphiboles and micas found in aquifers, which are primarily granitic-rich rocks. Longer residence time results in substantial water–rock interactions for fluoride mineral resolution [147]. In shallow groundwater, soluble salts are gradually reduced, pH is increased, and the dissolution fluorite equilibrium is observed in areas with high fluoride concentration [148]. The predominant hydrogeochemical mechanisms in groundwater are silicate weathering and ion exchange. Therefore, weathering and cation exchange influence the major groundwater chemistry, significantly affecting fluoride enrichment; an essential natural activity is controlling groundwater chemistry [149].
4.2. Soil
Fluoride in soil was found to be a mobile element. Furthermore, relative mobility suggested that soils played a more significant role in releasing fluoride into groundwater than rocks. Fluoride solubility in the soil is profoundly affected by soil pH, texture, organic matter, and concentration of other ions [171,172,173,174,175]. In soil, fluoride concentrations vary from 100 to 400 mg L−1 [13]; fluoride in soil is primarily combined with clay fraction of the soil colloids, and its mobility depends on the type of sorbents, pH, soil salinity, and sorption capacity [5,13,176]. For example, acidic soils can increase the bioavailability of fluoride; however, solubility decreases at pH in the range of 6–6.5 [171,172,177]. Fluoride generally combines with aluminum or calcium in the soil, so clay soil or silts have a higher fluoride concentration than sandy soil [178]. Fluoride mobility is affected at the high salinity; i.e., other ions in high concentration compete with fluoride for sorption sites [13]. Moreover, evapotranspiration can also regulate soil fluoride concentration and salinity [179]. Fluoride is mainly immobile in soil because it is not readily soluble and exchangeable, but soluble fluoride is vital to plant and animal growth [180,181].
5. Fluoride Uptake and Bioaccumulation in Plants and Foods
Fluoride is a common phytotoxic contaminant for plants [190]. Plant species absorb fluoride from water and soil via roots and air through leaves [190,191], accumulate fluoride in different parts of the plant, and acquire fluoride at a higher level than in the environment [192,193]. Fluoride accumulation in plants depends on various factors such as fluoride concentration in soil, plant species, and soil properties [178]. Fluoride accumulation in soil surrounds roots and disturbs the biochemical, morphological, and physiological behavior of plants. Plants grown in uncontaminated soils have an average fluoride concentration of less than 10 mg kg−1 [194]. Besides this, fluoride toxicity in plants depends on the level, frequency, and duration of exposure and the genotype of plants [1,195]. The presence of other anions and changes in pH also influences the fluoride accumulation in soil [131]. The growth and productivity of crop species and other plants are also affected detrimentally because of fluoride exposure, even at lower concentrations [196].
Figure 2 shows how plants uptake fluoride from contaminated soil and air, translocate it and accumulate it in cell walls. Plants are vulnerable to fluoride accumulation, and their growth and development process can be affected negatively even with a lower level of fluoride deposition [196]. However, plants such as Zea mays and Lupinus luteus were found fluoride-tolerant because of their ability of protein synthesis and self-protection against protein degradation [208].
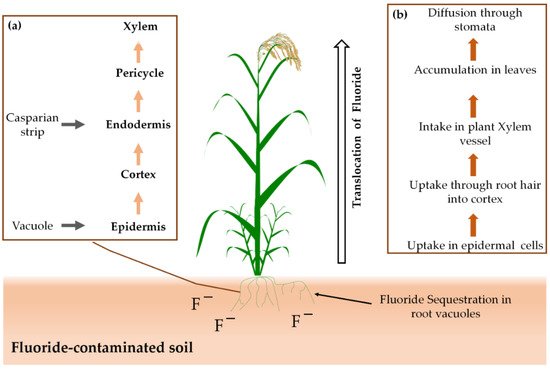
Figure 2. Fluoride uptake and accumulation in plants: (a) uptake mechanism in roots and (b) overall translocation from roots to shoots.
6. Microbial Remediation Techniques for Fluoride Removal
Microbial techniques are based upon using native or genetically modified microorganisms to remove fluoride ions from contaminated areas for environmental protection [103]. Different types of bacteria and other microorganisms rarely exhibited any toxic effect while exposed to high fluoride concentrations [213]. The antimicrobial effect of fluoride occurs by direct inhibition of enzymes by fluoride ion or hydrogen fluoride through the formation of phosphate analogs, such as alumino-fluoride or beryllium fluoride, that affect phosphate group transferring enzymes by inhibiting the adenosine triphosphate (ATP) synthesis. Microorganisms can perform various processes, such as mineralization, metal uptake, accumulation, sorption, enzymatic oxidation and reduction, extracellular precipitation, and efflux of xenobiotics using ionospheres to overcome fluoride toxicity [102,226].
This entry is adapted from the peer-reviewed paper 10.3390/pr9122154
This entry is offline, you can click here to edit this entry!
