
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marios Spanakis | + 2279 word(s) | 2279 | 2021-11-23 03:41:53 | | | |
| 2 | Amina Yu | -2 word(s) | 2277 | 2021-12-17 02:09:58 | | |
Video Upload Options
The absence of COVID-19-targeted treatments has led scientist to exploit available scientific evidence for potential efficient drugs that may block biological pathways of SARS-CoV-2 and several molecules have been emerged as promising pharmacological agents. Then again, due to the criticality of the disease, it is important for healthcare providers in COVID-19 clinics to recognize potential drug-drug interactions (DDIs) that may lead to adverse drug reactions (ADRs) and additional burdens in patients' health status from the administration of these agents.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease (COVID-19) is still pressing the healthcare systems worldwide to their limits [1]. Since its outbreak in 2019 [2][3], there are continuous efforts from the scientific community to develop efficient strategies [4][5]. The absence of targeted medicinal options and the spreading speed of the virus lead scientists to look for potential drugs through drug repurposing and use of available scientific data to block biological pathways of SARS-CoV-2 [6][7]. Since the COVID-19 pandemic crisis there are a lot of available epidemiological studies to determine COVID-19 frequency measures. It is well defined that morbidity and mortality rates are higher in elderly people with underlying chronic diseases, such as cardiovascular diseases (CVD); diabetes; respiratory disorders, such as chronic obstructive pulmonary disease (COPD); diabetes; and cancer [8][9].It is estimated that the overall fatality from COVID-19 in people with no underlying health problems is about 2–3%, while it is six- to ten-fold higher with people with one or more underlying health conditions. In this respect, given also that COVID-19 is referred to as an acute respiratory syndrome, it is of no surprise that underlying medical conditions that increase a person’s risk of severe illness from COVID-19 include respiratory disorders, such as COPD and asthma, cystic fibrosis etc. [10].
Numerous medications and medication protocols have been tested thus far, while several clinical trials are ongoing. Since most of the COVID-19-related hospitalizations refer to patients with underlying conditions that receive additional medications for their chronic treatments. Hence one of the most important clinical considerations is the potential and clinically significant drug-drug interactions (DDIs) that may lead to unwanted adverse drug reactions (ADRs) with additional negative impact on patients’ health status.
DDDIs occur when co-administered drugs share common biological pathways involved in their pharmacologic action. The result is a greater or lesser systemic exposure and pharmacologic action for one drug due to co-administration of another. DDIs are clinically significant when the modification in victim-drug exposure exceeds effective levels or reduced to sub-therapeutic concentrations depending on the perpetrator’s action as inhibitor or inducer. Synergistic or competitive actions on the victim’s drug primary or secondary biological targets and signaling pathways are characterized as PD-DDIs, while PK-DDIs occur when victim’s drug absorption, distribution, metabolism, and elimination (ADME) processes are altered [11].
DDIs represent a major issue in clinical practice since potential modulation of the clinical outcome may result in adverse drug reactions (ADRs), a harmful response from a system organ (or whole body) to a drug, which occurs at doses normally administered [12][13][14]. DDIs can be evaluated and categorized in relation to their severity as of: (i) major importance due to adequate clinical observations that evaluates the combinations as “serious—avoid” and/or “contraindicated”; (ii) of “use with caution—monitor” or “moderate” importance, where clinical evidence suggest co-administration can be considered after a risk/benefit analysis; and (iii) of “moderate-minor” or “minor” significance, where DDIs may be described in experimental level but are not clinically observed [11][15].
Within the entry, it discuss potentially clinically significant DDIs of therapies administered in COVID-19 with an emphasis on drugs for respiratory disorders, their underlying pharmacological mechanisms, potential ADRs, and how healthcare personnel should be aware to recognize and manage them. Scientific information for COVID-19 candidate treatments in based on dashboard available from DrugBank or other relative sources [16]. TThe anatomic therapeutic classification (ATC) was used to extract classified drugs for respiratory disorders (ATC-R) that are used in chronic respiratory diseases. The drug interaction checkers from Medscape and Drugs.com was used to search available scientific evidence, along with the summaries of product characteristics (SmPC) or product information for all drugs. The characterization of the clinical significance for DDIs was based on available scientific evidence as described in previous works [17][18][19][20].
2. COVID-19 Therapeutic Approaches and Drug Interactions
There are three primary pharmacological goals for treatment for COVID-19: (i) controlling of virus’s replication with antiviral drugs or drugs that modulate cellular mechanisms, which may play role in virus replication process (antimicrobials) ; (ii) supportive treatment for controlling of inflammation and immune response with glucocorticoids that are used in syndromes closely related to COVID-19 and/or with immunosuppressive agents, such as monoclonal antibodies; and (iii) reducing the risk for complications, such as thrombosis, with antiplatelet and aspirin as adjunct treatments[7][21][22][23]. Table 1 summarizes drugs that thus far have been emerged in COVID-19 [24], while their current level of evidence regarding their effectiveness on COVID-19 patients is depicted in Figure 1 . Table 2 summarizes ADRs that may occur in COVID-19 patients and are related with DDIs, which are also discussed. More information with examples of drug combinations and the potential clinical outcome can be found in the review entry and its supplementary files (Tables S1 and S2).
| Antivirals | Antimicrobials | Immunomodulators | Adjunct Agents |
|---|---|---|---|
| Atazanavir | Azithromycin | Anakinra | Aspirin |
| Darunavir | Chloroquine | Bamlanivimab | Bevacizumab |
| Fabifavir | Hydroxychloroquine | Baricitinib | Dalteparin |
| Lopinavir/Ritonavir | Ivermectin | Canakinumab | Enoxaparin |
| Remdesivir | Nitazoxanide | Colchicine | |
| Ribavirin | Dexamethasone | ||
| Fingolimod | |||
| Hydrocortisone | |||
| Methylprednisolone | |||
| Ruxolitinib | |||
| Sarilumab | |||
| Tocilizumab |

| COVID-19 Medications | Co-Medications | DDI Type | Significance | Clinical Signs-ADRs |
|---|---|---|---|---|
| Antivirals | HIV-AIDs | PK | Serious—Avoid or Monitor | Fatigue, irregular breathing, cold-blue hands/feet, nausea |
| Antineoplastic | PK | Serious—Avoid or Monitor | Tiredness, nausea, vomiting, anemia, etc. | |
| Immunosuppressants, | PK | Serious—Avoid or Monitor | Secondary infections, nausea, vomiting, tremors, etc. | |
| Antiarrhythmics/Ca2+ blockers | PK | Serious—Avoid or Monitor | Hypotension, edema, constipation, drowsiness, nausea, rash | |
| DOACs/anticoagulants | PK | Use with caution | Bleeding risk, bleeding signs | |
| SSRIs or TCAs | PK | Use with caution | Nausea, dizziness, hypotension, syncope | |
| Antipsychotics | PK | Serious—Avoid or Monitor | Cardiac arrhythmias | |
| Sedatives | PK | Serious—Avoid or Monitor | Prolonged sedation | |
| GI-track | PK | Serious—Avoid or Monitor | Cardiac arrhythmias | |
| Antihistamines | PK | Use with caution | Cardiac arrhythmias | |
| Antimicrobial | Anti-infectives (antibiotics, antifungals, antimalarials) | PD | Serious—Avoid or Monitor | QT-prolongation |
| GI-agents | PD | Serious—Avoid or Monitor | QT-prolongation | |
| Psychotropic (antipsychotics, SSRIs, TCAs) | PD | Serious—Avoid or Monitor | QT-prolongation | |
| Analgesics (opioids) | PD | Serious—Avoid or Monitor | QT-prolongation | |
| Antihistamines | PD | Serious—Avoid or Monitor | QT-prolongation | |
| Antiarrhythmics | PD | Serious—Avoid or Monitor | QT-prolongation | |
| Anesthetics | PD | Serious—Avoid or Monitor | QT-prolongation | |
| Antiepileptics (with chloroquine) | PD | Use with caution | Convulsions | |
| Immunomodulatory | DMARDs | PD | Serious—avoid or monitoring | prolonged pharmacological action, myelosuppression toxicity |
| TDM, narrow therapeutic index, increased first-pass effect drugs | PK | Use with caution | Increased metabolism modulation of steady-state and drug response | |
| Colchicine-CYP inhibitors | PK | Use with caution | Nausea, dizziness, cramping, pain, vomit |
DDI: drug-drug interactions; ADRs: adverse drug reactions; DOACs: direct oral anticoagulants; SSRIs: Selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants; DMARD: disease-modifying antirheumatic drugs; TDM: therapeutic drug monitoring; CYP: Cytochrome P-450.
Within the review, we focus on respiratory diseases, such as asthma, COPD, emphysema, cystic fibrosis, and bronchiectasis. These disorders are usually treated with systemic administration of drugs from ATC-R and mainly R03, drugs for obstructive airway diseases, through several biopharmaceutical products, such as inhalers, oral, or intravenous treatments. Table 3 summarizes the potential interactions and the ADRs that may be observed in COVID-19 patients and are related with DDIs when co-administered with treatment options for COVID-19. Additional information with examples of drug combinations of from ATC-R class with COVID-19 emerged treatments and the potential clinical outcome can be found in Supplementary File (Table S3) .
Table 3. DDIs mechanism and significance between drugs used in respiratory disorders and COVID-19 medications along with potential ADRs that may occur in those patients.
|
Respiratory Medications |
COVID-19 Medications |
DDI Type |
Significance |
Clinical Signs-ADRs |
|
β2-agonists |
chloroquine/hydroxychloroquine |
PD |
Use with caution |
QT prolongation |
|
antivirals |
PK |
Moderate |
prolonged pharmacological action-tachycardia, anxiety, tremor |
|
|
Glucocorticoids |
immunosuppressants (interleukin inhibitors) |
PD |
Moderate |
Neutropenia |
|
darunavir |
PK |
Use with caution |
Cushing’s syndrome |
|
|
Anticholinergics |
antivirals (lopinavir/ritonavir) |
PK |
Use with caution |
prolonged pharmacological action |
|
Xanthines |
immunosuppressants (interleukin inhibitors), antivirals |
PK |
Use with caution |
prolonged pharmacological action |
|
LTRA |
antivirals, chloroquine, hydroxychloroquine, immunosuppressants |
PD |
Use with caution |
Neutropenia |
|
remdesivir |
PD |
Moderate |
hepatotoxicity |
|
|
Antihistamine |
antivirals |
PK |
Moderate |
prolonged pharmacological action antihistamine related ADRs |
|
Antimicrobials (azithromycin, cholorquine, etc.) |
PD |
Use with caution |
QT prolongation |
3. Healthcare Personnel in COVID-19 Clinics: Identifying and Manage DDIs and ADRs
Recently, as this review is submitted, there was the announcement for molnupiravir, a nucleoside analogue that interferes within SARS-CoV-2’s RNA replication for which clinical trials of phase 3 are coming to an end, and the drug will seek emergency authorization license for patients at mild-to-moderate or high risk of developing severe COVID-19 [34]. Whatever the case may be, SARS-CoV-2 challenges scientists in academia and industry “benches” and puts to test the “bedside” of healthcare systems[35]. From the perspective for comorbidities in COVID-19 patients, COVID-19 is the common underlying disease for so many different clinical cases of patients with comorbidities and so many different clinical scenarios [36][37].
COVID-19 challenges the healthcare eco-system also on the awareness of healthcare teams to exploit their knowledge regarding optimum healthcare provision in complicate clinical scenarios[38]. This universality of the disease creates different and complicated clinical scenarios especially for infected patients with comorbidities and complex therapeutic schemes. Especially for chronic diseases for which patients follow a stable treatment to manage their condition (i.e., COPD, asthma, or other respiratory diseases), the complications of COVID-19 may raise new challenges and needs for therapy adjustments. Similar tasks may be raised for other cases of patients that receive chronic treatments, such as people with epilepsy or people with coagulation problems etc. [39][40][41][42][43][44]. Hence, it is crucial that healthcare professionals, especially those in COVID-19 clinics, be as much aware as possible regarding management of adverse drug events (ADEs), ADRS, and/or DDIs in order to ensure optimum healthcare provision [45][46][47][48][49][50][51]. Among healthcare professionals, clinical pharmacists can contribute significantly to the prevention, reduction, and management of drug-related problems, such as DDIs and ADRs, during patient hospitalization [52][53][54]. Their contribution in evaluating clinical scenarios for symptom assessment (including ADRs) and treatment decisions for optimized therapeutic schemes as well as patient education during hospitalization in COVID-19 recovery clinics has been already assessed [55][56][57][58][59][60].
Within the review, we present repurposed drugs for COVID-19 patients and discussing their potential for DDIs with a special focus on patients with respiratory disorders except lung cancer pharmacotherapy since these patients by default are a different category [10][47]. Advancing previous works, we present potential clinical signs that could be strongly related with ADRs from occurring DDIs and not with disease progression discussing ways to assess this cases [60][61][62]. One of the most often occurring ADRs is QT prolongation, which can also be a result of an occurring DDI. Although they seem to be the most prevalent, cardiac arrhythmias are not the only occurring ADRs that healthcare professionals should be aware of (Figure 2).
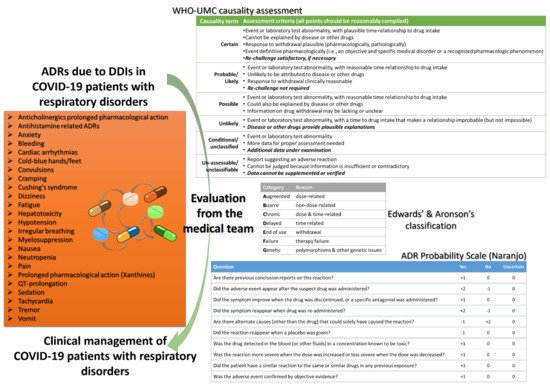
The management of ADRs and DDIs from healthcare personnel, especially doctors, clinical pharmacists, and nurses who work in COVID-19 and ICU clinics, is crucial for optimal healthcare provision. Recently, it was estimated that ~40% of COVID-19 patients are exposed to at least one clinically significant DDI with antiviral drugs, with protease inhibitors as the most often associated medications in DDIs, whereas the number of patients in other works reaches up to 60% [45][52][63]. As the scientific community is still “educating” itself regarding all the biological background of SARs-CoV-2, it is of high importance the pharmacovigilance procedures for the optimum healthcare management. It is crucial that any deviation from the clinical protocols for a patient diagnosed with COVID-19 should be evaluated and reported so to be further analyzed from the respective stakeholders (regulatory, authorities, etc.). Thus, all the gathered knowledge can be filtered through appropriate channels from pharmacovigilance regulatory bodies to be evaluated, revised, and return to enrich the clinical protocols (Figure 3). Such an active “pharmacovigilant” role of healthcare professionals will enhance also public confidence to healthcare systems against misinformation [65][66][67][68].
4. Conclusions
COVID-19 is here to stay. It does not discriminate health status and it is especially harsh for patient population groups with comorbidities, such as patients with respiratory disorders. Apart of the vaccination programs that try to restrain the speed of the pandemic waves through development of herd immunity and reduce the risk for severe acute respiratory syndrome, it is important to develop potential treatments for the those that will be eventually infected. A key issue is the awareness of healthcare professionals for optimization of healthcare provision and minimization of any ADEs, especially ADRs causally related with DDIs. For patients with respiratory disorders DDIs are mostly of moderate importance, however some cases of clinically significant outcomes exist, and special precautions should be considered. For treatment goal to be achieved, a stepwise approach should be followed, including prediction and avoidance of DDIs and ADRs and proper healthcare. This requires a medical team of physicians, clinical pharmacists, and nurses with high awareness of effective management of drug-related problems and capability of risk-benefit analysis of patients’ health status. Tools such as clinical decision support systems can assist in prioritization of actions, including optimum treatment options, for patients in high risk, such as those with respiratory disorders and comorbidities, as well as sophisticated clinical protocols that minimize ADRs and enhance therapeutic outcomes.
References
- WHO Coronavirus Disease (COVID-19). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 12 May 2021).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513.
- Nussbaumer-Streit, B.; Mayr, V.; Dobrescu, A.I.; Chapman, A.; Persad, E.; Klerings, I.; Wagner, G.; Siebert, U.; Christof, C.; Zachariah, C.; et al. Quarantine alone or in combination with other public health measures to control COVID-19: A rapid review. Cochrane Database Syst. Rev. 2020, 9.
- Zhou, A.; Sabatello, M.; Eyal, G.; Lee, S.S.J.; Rowe, J.W.; Stiles, D.F.; Swanson, A.; Appelbaum, P.S. Is precision medicine relevant in the age of COVID-19? Genet. Med. 2021, 23, 999–1000.
- Kim, M.S.; An, M.H.; Kim, W.J.; Hwang, T.H. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: A systematic review and network meta-analysis. PLoS Med. 2020, 17, e1003501.
- Scavone, C.; Mascolo, A.; Rafaniello, C.; Sportiello, L.; Trama, U.; Zoccoli, A.; Bernardi, F.F.; Racagni, G.; Berrino, L.; Castaldo, G.; et al. Therapeutic strategies to fight COVID-19: Which is the status artis? Br. J. Pharmacol. 2021, 1–21.
- Zhou, Y.; Yang, Q.; Chi, J.; Dong, B.; Lv, W.; Shen, L.; Wang, Y. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 99, 47–56.
- Du, P.; Li, D.; Wang, A.; Shen, S.; Ma, Z.; Li, X. A Systematic Review and Meta-Analysis of Risk Factors Associated with Severity and Death in COVID-19 Patients. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 1–12.
- Alkhathami, M.G.; Advani, S.M.; Abalkhail, A.A.; Alkhathami, F.M.; Alshehri, M.K.; Albeashy, E.E.; Alsalamah, J.A. Prevalence and mortality of lung comorbidities among patients with COVID-19: A systematic review and meta-analysis. Lung India 2021, 38, S31–S40.
- Hines, L.E.; Malone, D.C.; Murphy, J.E. Recommendations for generating, evaluating, and implementing drug-drug interaction evidence. Pharmacotherapy 2012, 32, 304–313.
- WHO International Drug Monitoring: The Role of the Hospital; World Health Organization Technical Report Series No. 425; World Health Organization: Geneva, Switzerland, 1969; pp. 5–24.
- Leétinier, L.; Ferreira, A.; Marceron, A.; Babin, M.; Micallef, J.; Miremont-Salameé, G.; Pariente, A.; on behalf of the French Network of Pharmacovigilance Centres. Spontaneous Reports of Serious Adverse Drug Reactions Resulting from Drug–Drug Interactions: An Analysis From the French Pharmacovigilance Database. Front. Pharmacol. 2021, 11, 624562.
- Edwards, I.R.; Aronson, J.K. Adverse drug reactions: Definitions, diagnosis, and management. Lancet 2000, 356, 1255–1259.
- Scheife, R.T.; Hines, L.E.; Boyce, R.D.; Chung, S.P.; Momper, J.D.; Sommer, C.D.; Abernethy, D.R.; Horn, J.R.; Sklar, S.J.; Wong, S.K.; et al. Consensus recommendations for systematic evaluation of drug-drug interaction evidence for clinical decision support. Drug Saf. 2015, 38, 197–206.
- Wishart, D.S. DrugBank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672.
- Spanakis, M.; Roubedaki, M.; Tzanakis, I.; Zografakis-Sfakianakis, M.; Patelarou, E.; Patelarou, A. Impact of adverse drug reactions in patients with end stage renal disease in Greece. Int. J. Environ. Res. Public Health 2020, 17, 9101.
- Spanakis, M.; Melissourgaki, M.; Lazopoulos, G.; Patelarou, A.E.; Patelarou, E. Prevalence and clinical significance of drug–drug and drug–dietary supplement interactions among patients admitted for cardiothoracic surgery in greece. Pharmaceutics 2021, 13, 239.
- Spanakis, M.; Sfakianakis, S.; Kallergis, G.; Spanakis, E.G.; Sakkalis, V. PharmActa: Personalized pharmaceutical care eHealth platform for patients and pharmacists. J. Biomed. Inform. 2019, 100, 103336.
- Spanakis, M.; Sfakianakis, S.; Sakkalis, V.; Spanakis, E.G. PharmActa: Empowering Patients to Avoid Clinical Significant Drug(-)Herb Interactions. Medicines 2019, 6, 26.
- Center for Disease Control and Prevention. Information for Clinicians on Investigational Therapeutics for Patients with COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html (accessed on 27 July 2021).
- Trivedi, N.; Verma, A.; Kumar, D. Possible treatment and strategies for COVID-19: Review and assessment. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12593–12608.
- Khani, E.; Khiali, S.; Entezari-Maleki, T. Potential COVID-19 Therapeutic Agents and Vaccines: An Evidence-Based Review. J. Clin. Pharmacol. 2021, 61, 429–460.
- Potì, F.; Pozzoli, C.; Adami, M.; Poli, E.; Costa, L.G. Treatments for COVID-19: Emerging drugs against the coronavirus. Acta Bio-Med. Atenei Parm. 2020, 91, 118.
- Romark Pharmaceuticals. Alinia ® (Nitazoxanide) Tablets (Nitazoxanide) for Oral Suspension. 2005. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021818lbl.pdf (accessed on 3 August 2021).
- Blum, V.F.; Cimerman, S.; Hunter, J.R.; Tierno, P.; Lacerda, A.; Soeiro, A.; Cardoso, F.; Bellei, N.C.; Maricato, J.; Mantovani, N.; et al. Nitazoxanide superiority to placebo to treat moderate COVID-19—A Pilot prove of concept randomized double-blind clinical trial. EClinicalMedicine 2021, 37, 100981.
- Zhang, Y.; Zhong, Y.; Pan, L.; Dong, J. Treat 2019 novel coronavirus (COVID-19) with IL-6 inhibitor: Are we already that far? Drug Discov. Ther. 2020, 14, 100–102.
- WHO Recommends Life-Saving Interleukin-6 Receptor Blockers for COVID-19 and Urges Producers to Join Efforts to Rapidly Increase Access. Available online: https://www.who.int/news/item/06-07-2021-who-recommends-life-saving-interleukin-6-receptor-blockers-for-covid-19-and-urges-producers-to-join-efforts-to-rapidly-increase-access (accessed on 3 August 2021).
- FDA. Kineret® (Anakinra) for Injection, for Subcutaneous Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/103950s5175lbl.pdf (accessed on 3 August 2021).
- EMA-CHMP. Ilaris Annex I Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ilaris (accessed on 3 August 2021).
- Roche. Actemra (tocilizumab) PRODUCT MONOGRAPH. 2010. Available online: https://www.rochecanada.com/PMs/Actemra/Actemra_PM_E.pdf (accessed on 27 August 2021).
- Kim, S.; Östör, A.J.K.; Nisar, M.K. Interleukin-6 and cytochrome-P450, reason for concern? Rheumatol. Int. 2012, 32, 2601–2604.
- Pulciani, S.; Di Lonardo, A.; Fagnani, C.; Taruscio, D. P4 Medicine versus Hippocrates. Ann. Ist. Super Sanita 2017, 53, 185–191.
- Loucera, C.; Esteban-Medina, M.; Rian, K.; Falco, M.M.; Dopazo, J.; Peña-Chilet, M. Drug repurposing for COVID-19 using machine learning and mechanistic models of signal transduction circuits related to SARS-CoV-2 infection. Signal Transduct. Target. Ther. 2020, 5, 1–3.
- Banerjee, A.K.; Arora, N. Coronavirus Disease (COVID-19) Pandemic: A Race Against Time. Curr. Top. Med. Chem. 2020, 20, 1434–1437.
- Ng, W.H.; Tipih, T.; Makoah, N.A.; Vermeulen, J.-G.; Goedhals, D.; Sempa, J.B.; Burt, F.J.; Taylor, A.; Mahalingam, S. Comorbidities in SARS-CoV-2 Patients: A Systematic Review and Meta-Analysis. Mbio 2021, 12, 1–12.
- Willyard, C. How antiviral pill molnupiravir shot ahead in the COVID drug hunt. Nature 2021.
- Arabi, Y.M.; Azoulay, E.; Al-Dorzi, H.M.; Phua, J.; Salluh, J.; Binnie, A.; Hodgson, C.; Angus, D.C.; Cecconi, M.; Du, B.; et al. How the COVID-19 pandemic will change the future of critical care. Intensive Care Med. 2021, 47, 282–291.
- FDA. Plaquenil ® Hydroxychloroquine Sulfate Tablets, USP Description. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/009768s037s045s047lbl.pdf (accessed on 3 August 2021).
- Sanofi. ARALEN® CHLOROQUINE PHOSPHATE, USP. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/006002s044lbl.pdf (accessed on 3 August 2021).
- Makowsky, M.J.; Schindel, T.J.; Rosenthal, M.; Campbell, K.; Tsuyuki, R.T.; Madill, H.M. Collaboration between pharmacists, physicians and nurse practitioners: A qualitative investigation of working relationships in the inpatient medical setting. J. Interprof. Care 2009, 23, 169–184.
- Rider, F.K.; Lebedeva, A.V.; Mkrtchyan, V.R.; Guekht, A.B. Epilepsy and covid-19: Patient management and optimization of antiepileptic therapy during pandemic. Zhurnal Nevrol. I Psikhiatrii Im. SS Korsakova 2020, 120, 100–107.
- Karaźniewicz-Łada, M.; Główka, A.K.; Mikulska, A.A.; Główka, F.K. Pharmacokinetic Drug-Drug Interactions among Antiepileptic Drugs, Including CBD, Drugs Used to Treat COVID-19 and Nutrients. Int. J. Mol. Sci. 2021, 22, 9582.
- Asadi-Pooya, A.A.; Attar, A.; Moghadami, M.; Karimzadeh, I. Management of COVID-19 in people with epilepsy: Drug considerations. Neurol. Sci. 2020, 41, 2005–2011.
- Mahboobipour, A.A.; Baniasadi, S. Clinically important drug–drug interactions in patients admitted to hospital with COVID-19: Drug pairs, risk factors, and management. Drug Metab. Pers. Ther. 2021, 36, 9–16.
- Michaud, V.; Dow, P.; Al Rihani, S.B.; Deodhar, M.; Arwood, M.; Cicali, B.; Turgeon, J. Risk Assessment of Drug-Induced Long QT Syndrome for Some COVID-19 Repurposed Drugs. Clin. Transl. Sci. 2021, 14, 20–28.
- Bajgain, K.T.; Badal, S.; Bajgain, B.B.; Santana, M.J. Prevalence of comorbidities among individuals with COVID-19: A rapid review of current literature. Am. J. Infect. Control 2021, 49, 238–246.
- Baburaj, G.; Thomas, L.; Rao, M. Potential Drug Interactions of Repurposed COVID-19 Drugs with Lung Cancer Pharmacotherapies. Arch. Med. Res. 2021, 52, 261–269.
- Lemaitre, F.; Solas, C.; Grégoire, M.; Lagarce, L.; Elens, L.; Polard, E.; Saint-Salvi, B.; Sommet, A.; Tod, M.; Guellec, C.B.-L. Potential drug–drug interactions associated with drugs currently proposed for COVID-19 treatment in patients receiving other treatments. Fundam. Clin. Pharmacol. 2020, 34, 530–547.
- Godino, C.; Scotti, A.; Maugeri, N.; Mancini, N.; Fominskiy, E.; Margonato, A.; Landoni, G. Antithrombotic therapy in patients with COVID-19? -Rationale and Evidence-. Int. J. Cardiol. 2021, 324, 261.
- Rezaee, H.; Pourkarim, F.; Pourtaghi-Anvarian, S.; Entezari-Maleki, T.; Asvadi-Kermani, T.; Nouri-Vaskeh, M. Drug-drug interactions with candidate medications used for COVID-19 treatment: An overview. Pharmacol. Res. Perspect. 2021, 9, e00705.
- Martínez-López-de-Castro, N.; Samartín-Ucha, M.; Paradela-Carreiro, A.; Pérez-Landeiro, A.; Inaraja-Bobo, M.T.; Álvarez-Payero, M.; Castro-Núñez, I.; García-Beloso, N.; Robles-Torres, D.; López-López, A.; et al. Real-world prevalence and consequences of potential drug-drug interactions in the first-wave COVID-19 treatments. J. Clin. Pharm. Ther. 2021, 46, 724–730.
- Francis, J.; Abraham, S. Clinical pharmacists: Bridging the gap between patients and physicians. Saudi Pharm. J. 2014, 22, 600–602.
- Chou, Y.C.; Dang, V.T.; Yen, H.Y.; Lai, K.M. Influence of risk of drug–drug interactions and time availability on patient trust, satisfaction, and cooperation with clinical pharmacists. Int. J. Environ. Res. Public Health 2019, 16, 1566.
- Lopez-Martin, C.; Garrido Siles, M.; Alcaide-Garcia, J.; Faus Felipe, V. Role of clinical pharmacists to prevent drug interactions in cancer outpatients: A single-centre experience. Int. J. Clin. Pharm. 2014, 36, 1251–1259.
- Gross, A.E.; MacDougall, C. Roles of the clinical pharmacist during the COVID-19 pandemic. J. Am. Coll. Clin. Pharm. 2020, 3, 564–566.
- Arredondo, E.; Udeani, G.; Horseman, M.; Hintze, T.D.; Surani, S. Role of Clinical Pharmacists in Intensive Care Units. Cureus 2021, 13, e17929.
- Wang, D.; Liu, Y.; Zeng, F.; Shi, C.; Cheng, F.; Han, Y.; Zhang, Y. Evaluation of the role and usefulness of clinical pharmacists at the Fangcang Hospital during COVID-19 outbreak. Int. J. Clin. Pract. 2021, 75, e14271.
- Perez, M.; Masse, M.; Deldicque, A.; Beuscart, J.B.; De Groote, P.; Desbordes, J.; Fry, S.; Musy, E.; Odou, P.; Puisieux, F.; et al. Analysis of clinical pharmacist interventions in the COVID-19 units of a French university hospital. Eur. J. Hosp. Pharm. 2021, 1–6.
- Ward, S.; O’Reilly, R.; Crawford, P. Evaluating clinical pharmacist involvement in a COVID-19 intensive care recovery clinic. Pharm. J. 2021, 306, 7948.
- WHO-UMC. The Use of the WHO-UMC System for Standardised Case Causality Assessment. Available online: http://who-umc.org/Graphics/24734 (accessed on 5 September 2021).
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245.
- Saber-Moghaddam, N.; Hejazi, S.; Elyasi, S. Potential Drug–Drug Interactions among Hospitalized COVID-19 Patients Admitted to Medical Wards of a Referral Hospital, North-East of Iran: A Cross Sectional Study. J. Pharm. Care 2021, 9, 88–95.
- Preskorn, S.H.; Quadri, S. Why Are Patients With COVID-19 at Risk for Drug-Drug Interactions? J. Psychiatr. Pract. 2020, 26, 485–492.
- Najafi, S. Importance of Pharmacovigilance and the Role of Healthcare Professionals. J. Pharmacovigil 2018, 6, 1–2.
- Tuccori, M.; Convertino, I.; Ferraro, S.; Cappello, E.; Valdiserra, G.; Focosi, D.; Blandizzi, C. The Impact of the COVID-19 “Infodemic” on Drug-Utilization Behaviors: Implications for Pharmacovigilance. Drug Saf. 2020, 43, 699–709.
- Potts, J.; Genov, G.; Segec, A.; Raine, J.; Straus, S.; Arlett, P. Improving the Safety of Medicines in the European Union: From Signals to Action. Clin. Pharmacol. Ther. 2020, 107, 521–529.
- Spanakis, M.; Patelarou, A.E.; Patelarou, E. Nursing Personnel in the Era of Personalized Healthcare in Clinical Practice. J. Pers. Med. 2020, 10, 56.




