
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lucas Hilário Sousa | + 2750 word(s) | 2750 | 2021-12-03 02:32:49 | | | |
| 2 | Jessie Wu | Meta information modification | 2750 | 2021-12-16 03:30:01 | | |
Video Upload Options
In this entry, information about the common metabolites of the Callyspongia genus were grouped, as well as studies of the biological activity of these compounds. Through NMR data, 212 metabolites were identified from genus Callyspongia (15 species and Callyspongia sp.), belonging to classes such as polyacetylenes, terpenoids, steroids, alkaloids, polyketides, simple phenols, phenylpropanoids, nucleosides, cyclic peptides, and cyclic depsipep-tides. A total of 109 molecules have been reported with bioactive activity, mainly cytotoxic and antimicrobial (antibacterial and antifungal) action.
1. Introduction
The genus Callyspongia (Callyspongiidae) encompasses a group of demosponges including 261 described species, of which approximately 180 have been accepted after taxonomic reviews. The marine organisms of Callyspongia are distributed in tropical ecosystems, especially in the central and western Pacific, but also in the regions of the Indian, the West Atlantic, and the East Pacific Oceans. The reason for the interest in the genus Callyspongia is related to its potential production of bioactive compounds. In this review, we group the chemical information about the metabolites isolated from the genus Callyspongia, as well as studies of the biological activity of these compounds. Through NMR data, 212 metabolites were identified from genus Callyspongia (15 species and Callyspongia sp.), belonging to classes such as polyacetylenes, terpenoids, steroids, alkaloids, polyketides, simple phenols, phenylpropanoids, nucleosides, cyclic peptides, and cyclic depsipeptides. A total of 109 molecules have been reported with bioactive activity, mainly cytotoxic and antimicrobial (antibacterial and antifungal) action.
2. Chemical Aspects of Callyspongia species
2.1. Polyacetylenes
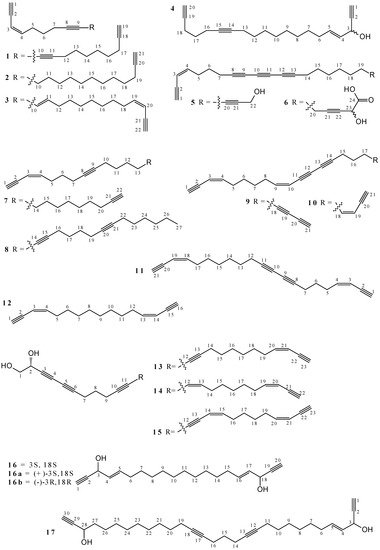
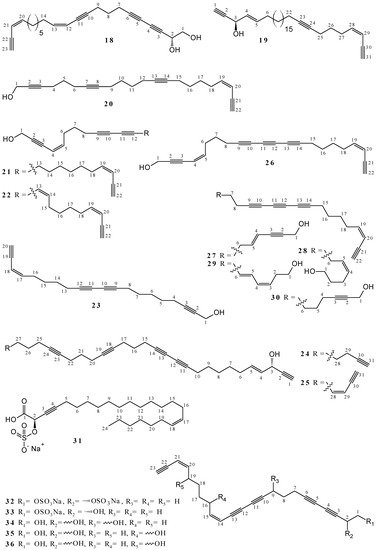
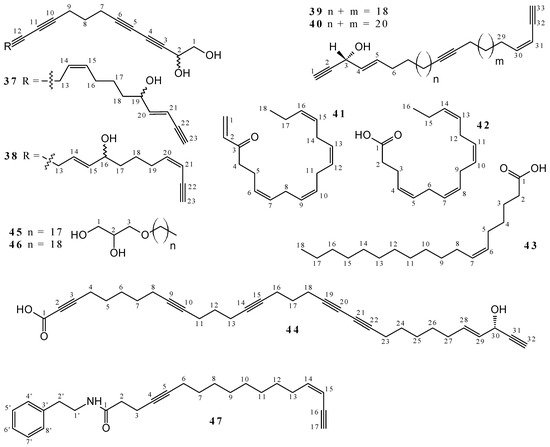
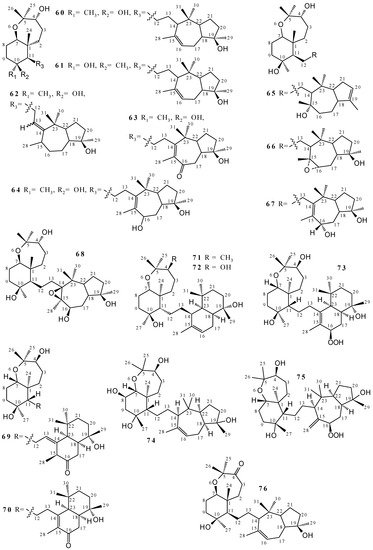
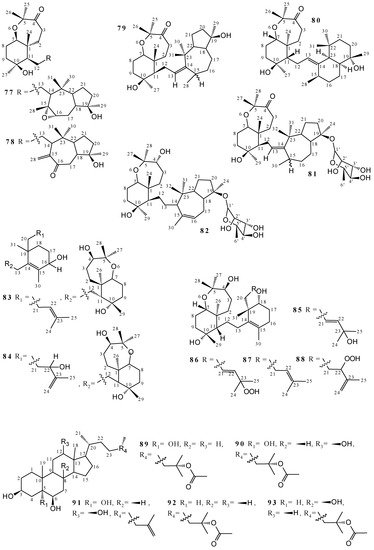
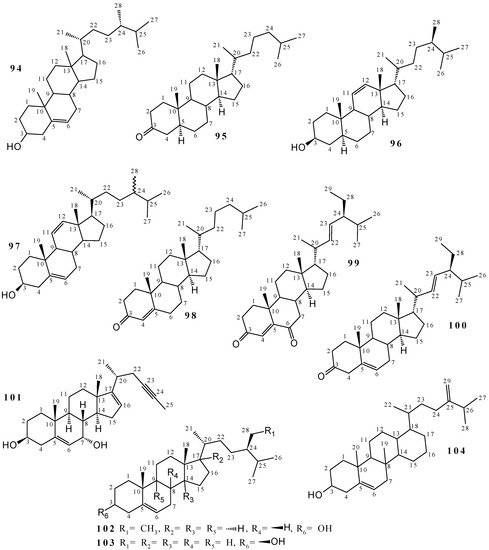
2.2. Terpenoids and Steroids
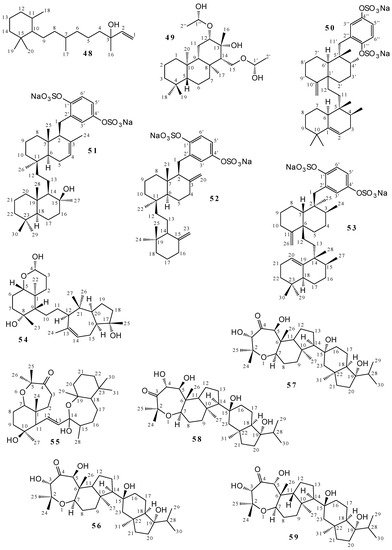
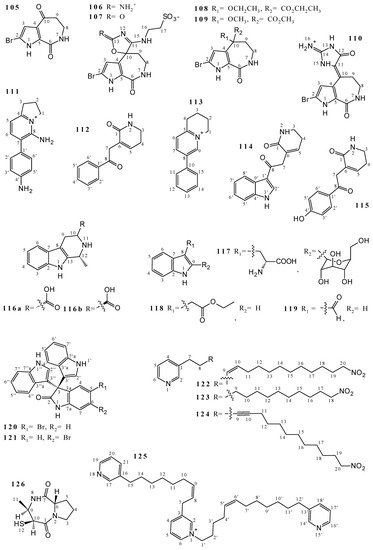
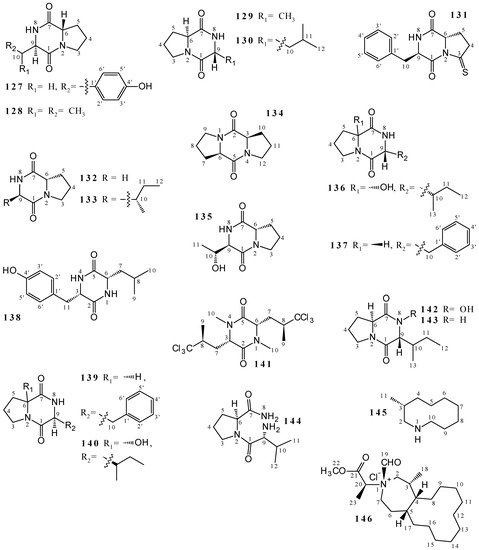
2.3. Alkaloids
3. Biological Aspects of Metabolites Isolated in Callyspongia species
3.1. Polyacetylenes
3.2. Terpenoids and Steroids
References
- Youssef, D.T.A.; Yoshida, W.Y.; Kelly, M.; Scheuer, P.J. Polyacetylenes from a red sea sponge Callyspongia species. J. Nat. Prod. 2000, 63, 1406–1410.
- Abdelmohsen, U.R.; Cheng, C.; Reimer, A.; Kozjak-Pavlovic, V.; Ibrahim, A.K.; Rudel, T.; Hentschel, U.; Edrada-Ebel, R.; Ahmed, S.A. Antichlamydial sterol from the red sea sponge Callyspongia aff implexa. Planta Med. 2015, 81, 382–387.
- Umeyama, A.; Nagano, C.; Arihara, S. Three novel C21 polyacetylenes from the marine sponge Callyspongia sp. J. Nat. Prod. 1997, 60, 131–133.
- Tsukamoto, S.; Kato, H.; Hirota, H.; Fusetani, N. Seven new polyacetylene derivatives, showing both potent metamorphosis-inducing activity in ascidian larvae and antifouling activity against barnacle larvae, from the marine Sponge Callyspongia truncata. J. Nat. Prod. 1997, 60, 126–130.
- Miao, S.; Andersen, R.J. Callydiyne, a new diacetylenic hydrocarbon from the sponge Callyspongla flammea. J. Nat. Prod. 1991, 54, 1433–1434.
- Umeyama, A.; Matsuoka, N.; Mine, R.; Nakata, A.; Arimoto, E.; Matsui, M.; Shoji, N.; Arihara, S.; Takei, M.; Hashimoto, T. Polyacetylene diols with antiproliferative and driving Th1 polarization effects from the marine sponge Callyspongia sp. J. Nat. Med. 2010, 64, 93–97.
- Fusetani, N.; Sugano, M.; Matsunaga, S.; Hashimoto, K. H,K-atpase inhibitors from the marine sponge Siphonochalina truncata: Absolute configuration of siphonodiol and two related metabolites. Tetrahedron Lett. 1987, 28, 4311–4312.
- Tada, H.; Yasuda, F. Siphonodiol, a new polyacetylenic metabolite from the sponge Siphonochalina truncate. Chem. Lett. 1984, 13, 779–780.
- Braekman, J.C.; Daloze, D.; Devijver, C.; Dubut, D.; Soest, R.W.M. A new C-20 polyacetylene from the sponge Callyspongia pseudoreticulata. J. Nat. Prod. 2003, 66, 871–872.
- Shirouzu, T.; Watari, K.; Ono, M.; Koizumi, K.; Saiki, I.; Tanaka, C.; Soest, R.W.M.; Miyamoto, T. Structure, synthesis, and biological activity of a C-20 bisacetylenic alcohol from a marine sponge Callyspongia sp. J. Nat. Prod. 2013, 76, 1337–1342.
- Sobahi, T.R.A.; Ayyad, S.E.N.; Abdel-Lateff, A.; Algandaby, M.M.; Alorfi, H.S.; Abdel-Naim, A.B. Cytotoxic metabolites from Callyspongia siphonella display antiproliferative activity by inducing apoptosis in HCT-116 cells. Pharmacogn. Mag. 2017, 13, 37–40.
- Ayyad, S.E.N.; Angawy, R.; Alarif, W.M.; Saqer, E.A.; Badria, F.A. Cytotoxic polyacetylenes from the red sea sponge Siphonochalina siphonella. Z. Nat. C 2014, 69, 117–123.
- Balansa, W.; Trianto, A.; Voogd, N.J.; Tanaka, J. A new cytotoxic polyacetylenic alcohol from a sponge Callyspongia sp. Nat. Prod. Commun. 2017, 12, 1909–1911.
- Youssef, D.T.A.; Soest, R.W.M.; Fusetani, N. Callyspongenols A-C, new cytotoxic C22-polyacetylenic alcohols from a red sea sponge, Callyspongia species. J. Nat. Prod. 2003, 66, 679–681.
- Chiu, C.W.; Su, H.J.; Lu, M.C.; Wang, W.H.; Sheu, J.H.; Su, J.H. Cytotoxic polyacetylenes from a formosan marine sponge Callyspongia sp. Bull. Chem. Soc. Jpn. 2014, 87, 1231–1234.
- Ki, D.W.; El-Desoky, A.H.; Wong, C.P.; Abdel-Ghani, M.; El-Beih, A.A.; Mizuguchi, M.; Morita, H. New cytotoxic polyacetylene alcohols from the egyptian marine sponge Siphonochalina siphonella. J. Nat. Med. 2020, 74, 409–414.
- Fujita, M.; Nakao, Y.; Matsunaga, S.; Soest, R.W.M.; Itoh, Y.; Seiki, M.; Fusetani, N. Callysponginol sulfate A, an MT1-MMP inhibitor isolated from the marine sponge Callyspongia truncata. J. Nat. Prod. 2003, 66, 569–571.
- Uno, M.; Ohta, S.; Ohta, E.; Ikegami, S. Callyspongins A and B: Novel polyacetylene sulfates from the marine sponge Callyspongia truncata that inhibit fertilization of starfish gametes. J. Nat. Prod. 1996, 59, 1146–1148.
- Rooney, F.; Capon, R.J. Callyspongynes A and B: New polyacetylenic lipids from a southern Australian marine sponge, Callyspongia sp. Lipids 1998, 33, 639–642.
- Urban, S.; Capon, R.J. A New lipid from an australian marine sponge, Callyspongia sp. Lipids 1997, 32, 675–677.
- El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Hassan, H.M.; Rateb, M.E.; Amin, E.; Mohammed, T.A.; El-Mesery, M.; Muhsinah, A.B.; Alsayari, A.; et al. Bioactive brominated oxindole alkaloids from the red sea sponge Callyspongia siphonella. Mar. Drugs 2019, 17, 465.
- Nakao, Y.; Uehara, T.; Matunaga, S.; Fusetani, N.; Soest, R.W.M. Callyspongynic acid, a polyacetylenic acid which inhibits α-glucosidase, from the marine sponge Callyspongia truncata. J. Nat. Prod. 2002, 65, 922–924.
- Xiao-Jian, L.; Shi-Hai, X.; Qi-Chang, H.; Dong-Hong, H. Studies on chemical constituents from Callyspongia fibrosa. Chin. J. Spectrosc. Lab. 2005, 22, 281–283.
- Rao, T.S.P.; Sarma, N.S.; Murthy, Y.L.N.; Kantamreddi, V.S.S.N.; Wright, C.W.; Parameswaran, P.S. New polyhydroxy sterols from the marine sponge Callyspongia fibrosa (Ridley & Dendly). Tetrahedron Lett. 2010, 51, 3583–3586.
- Youssef, D.T.A.; Soest, R.W.M.; Fusetani, N. Callyspongamide A, a new cytotoxic polyacetylenic amide from the red sea sponge Callyspongia fistularis. J. Nat. Prod. 2003, 66, 861–862.
- Rotem, M.; Kashman, Y. New polyacetylenes from the sponge Siphonochalina sp. Tetrahedron Lett. 1979, 20, 3193–3196.
- Delbeke, E.I.P.; Everaert, J.; Uitterhaegen, E.; Verweire, S.; Verlee, A.; Talou, T.; Soetaert, W.; Bogaert, I.N.A.; Stevens, C.V. Petroselinic acid purification and its use for the fermentation of new sophorolipids. AMB Express 2016, 6, 28.
- Garg, H.S.; Agraval, S. Callyspinol, a new diterpene from sponge Callyspongia spinossima. Tetrahedron Lett. 1995, 36, 9035–9038.
- Kurnianda, V.; Faradilla, S.; Karina, S.; Agustina, S.; Ulfah, M.; Octavina, C.; Syahliza, F.; Ramadhan, M.R.; Purnawan, S.; Musman, M. Polyoxygenated diterpene produced by the indonesian marine sponge Callyspongia sp. as an inhibitor of the human pancreatic cancer cells. Microbiol. Indones. 2019, 13, 70–74.
- Fukami, A.; Ikeda, Y.; Kondo, S.; Naganawa, H.; Takeuchi, T.; Furuya, S.; Hirabayashi, Y.; Shimoike, K.; Hosaka, S.; Watanabe, Y.; et al. Akaterpin, a novel bioactive triterpene from the marine sponge Callyspongia sp. Tetrahedron Lett. 1997, 38, 1201–1202.
- Gray, C.A.; Lira, S.P.; Silva, M.; Pimenta, E.F.; Thiemann, O.H.; Oliva, G.; Hajdu, E.; Andersen, R.J.; Berlinck, R.G.S. Sulfated meroterpenoids from the brazilian sponge Callyspongia sp. are inhibitors of the antileishmaniasis target adenosine phosphoribosyl transferase. J. Org. Chem. 2006, 71, 8685–8690.
- Jain, S.; Abraham, I.; Carvalho, P.; Kuang, Y.H.; Shaala, L.A.; Youssef, D.T.A.; Avery, M.A.; Chen, Z.S.; Sayed, K.A. Sipholane triterpenoids: Chemistry, reversal of ABCB1/P-glycoprotein-mediated multidrug resistance, and pharmacophore modeling. J. Nat. Prod. 2009, 72, 1291–1298.
- Kashman, Y.; Yosief, T.; Carmeli, S. New triterpenoids from the red sea sponge Siphonochalina siphonella. J. Nat. Prod. 2001, 64, 175–180.
- Carmely, S.; Kashman, Y. Neviotine-A, a new triterpene from the red sea sponge Siphonochalina siphonella. J. Org. Chem. 1986, 51, 784–788.
- Ayyad, S.E.N.; Angawi, R.F.; Saqer, E.; Abdel-Lateff, A.; Badria, F.A. Cytotoxic neviotane triterpene-type from the red sea sponge Siphonochalina siphonella. Pharmacogn. Mag. 2014, 10, 334–341.
- Al-Massarani, S.M.; El-Gamal, A.A.; Al-Said, M.S.; Al-Lihaibi, S.S.; Basoudan, O.A. In vitro cytotoxic, antibacterial and antiviral activities of triterpenes from the red sea sponge, Siphonochalina siphonella. Trop. J. Pharm. Res. 2015, 14, 33–40.
- El-Beih, A.A.; El-Desoky, A.H.; Al-hammady, M.A.; Elshamy, A.I.; Hegazy, M.E.F.; Kato, H.; Tsukamoto, S. New inhibitors of RANKL-induced osteoclastogenesis from the marine sponge Siphonochalina siphonella. Fitoterapia 2018, 128, 43–49.
- Shmueli, U.; Carmely, S.; Groweiss, A.; Kashman, Y. Sipholenol and Sipholenone, two new triterpenes from the marine sponge Siphonochalina Siphonella. Tetrahedron Lett. 1981, 22, 709–712.
- Jain, S.; Shirode, A.; Yacoub, S.; Barbo, A.; Sylvester, P.W.; Huntimer, E.; Halaweish, F.; Sayed, K.A. Biocatalysis of the anticancer sipholane triterpenoids. Planta Med. 2007, 73, 591–596.
- Jain, S.; Laphookhieo, S.; Shi, Z.; Fu, L.W.; Akiyama, S.I.; Chen, Z.S.; Youssef, D.T.A.; Soest, R.W.M.; Sayed, K.A. Reversal of P-glycoprotein-mediated multidrug resistance by sipholane triterpenoids. J. Nat. Prod. 2007, 70, 928–931.
- Al-Lhaibi, S.S.; Abdel-Lateff, A.; Alarif, W.M.; Nogata, Y.; Ayyad, S.E.N.; Okino, T. Potent antifouling metabolites from red sea organisms. Asian J. Chem. 2015, 27, 2252–2256.
- Carmely, S.; Kashman, Y. The sipholanes: A novel group of triterpenes from the marine sponge Siphonochalina siphonella. J. Org. Chem. 1983, 48, 3517–3525.
- Carmely, S.; Loya, Y.; Kashman, Y. Siphonellinol, a new triterpene from the marine sponge Siphonochalina siphonella. Tetrahedron Lett. 1983, 24, 3673–3676.
- Alam, P.; Alqahtani, A.S.; Husain, F.M.; Rehman, M.T.; Alajmi, M.F.; Noman, O.M.; Gamal, A.A.; Al-Massarani, S.M.; Khan, M.S. Siphonocholin isolated from red sea sponge Siphonochalina siphonella attenuates quorum sensing controlled virulence and biofilm formation. Saudi Pharm. J. 2020, 28, 1383–1391.
- Carmely, S.; Kashman, Y. The study of sipholanes by two-dimensional NMR spectroscopy. Magn. Reson. Chem. 1986, 24, 332–336.
- Youssef, D.T.A.; Ibrahim, A.K.; Khalifa, S.I.; Mesbah, M.K.; Mayer, A.M.S.; Soest, R.W.M. New Anti-inflammatory sterols from the red sea sponges Scalarispongia aqabaensis and Callyspongia siphonella. Nat. Prod. Commun. 2010, 5, 27–31.
- Plisson, F.; Prasad, P.; Xiao, X.; Piggott, A.M.; Huang, X.C.; Khalil, Z.; Capon, R.J. Callyspongisines A-D: Bromopyrrole alkaloids from an australian marine sponge, Callyspongia sp. Org. Biomol. Chem. 2014, 12, 1579–1584.
- Yang, B.; Tao, H.; Zhou, X.; Lin, X.P.; Liu, Y. Two new alkaloids from marine sponge Callyspongia sp. Nat. Prod. Res. 2012, 27, 1–5.
- Yang, B.; Huang, J.; Lin, X.; Zhang, Y.; Tao, H.; Liu, Y. A new diketopiperazine from the marine sponge Callyspongia species. Rec. Nat. Prod. 2016, 10, 117–121.
- Yang, B.; Hu, J.; Lei, H.; Chen, X.Q.; Zhou, X.F.; Liu, Y.H. Chemical constituents of marine sponge Callyspongia sp. from the south China sea. Chem. Nat. Compd. 2012, 48, 350–351.
- Wang, G.Y.S.; Kuramoto, M.; Uemura, D.; Yamada, A.; Yamaguchi, K.; Yazawa, K. Three Novel Anti-mierofouling nitroalkyl pyridine alkaloids from the Okinawan marine sponge Callyspongia sp. Tetrahedron Lett. 1996, 37, 1813–1816.
- Buchanan, M.S.; Carroll, A.R.; Addepalli, R.; Avery, V.M.; Hooper, J.N.A.; Quinn, R.J. Niphatoxin C, a cytotoxic tripyridine alkaloid from Callyspongia sp. J. Nat. Prod. 2007, 70, 2040–2041.
- Huang, R.M.; Ma, W.; Dong, J.D.; Zhou, X.F.; Xu, T.; Lee, K.J.; Yang, X.; Xu, S.H.; Liu, Y. A new 1,4-diazepine from south China sea marine sponge Callyspongia species. Molecules 2010, 15, 871–877.
- Kapojos, M.M.; Abdjul, D.B.; Yamazaki, H.; Ohshiro, T.; Rotinsulu, H.; Wewengkang, D.S.; Sumilat, D.A.; Tomoda, H.; Namikoshi, M.; Uchida, R. Callyspongiamides A and B, sterol O-acyltransferase inhibitors, from the Indonesian marine sponge Callyspongia sp. Bioorg. Med. Chem. Lett. 2018, 28, 1911–1914.
- Yang, B.; Dong, J.; Zhou, X.; Yang, X.; Lee, K.J.; Wang, L.; Zhang, S.; Liu, Y. Proline-containing dipeptides from a marine sponge of a Callyspongia Species. Helv. Chim. Acta. 2009, 92, 1112–1117.
- Chen, Y.; Peng, Y.; Gao, C.; Huang, R. A new diketopiperazine from South China Sea marine sponge Callyspongia sp. Nat. Prod. Res. 2014, 28, 1010–1014.
- Sperry, S.; Crews, P. A novel alkaloid from the indo-pacific sponge Clathria basilana. Tetrahedron Lett. 1996, 37, 2389–2390.
- Gopichand, Y.; Schmitz, F.J. Two novel lactams from the marine sponge Halichondria melanodocia. J. Org. Chem. 1979, 44, 4995–4997.
- Jayatilake, G.S.; Thornton, M.P.; Leonard, A.C.; Grimwade, J.E.; Baker, B.J. Metabolites from an antarctic sponge-associated bacterium, Pseudomonas aeruginosa. J. Nat. Prod. 1996, 59, 293–296.
- Adamczeski, M.; Reed, A.R.; Crews, P. New and known diketopiperazines from the Caribbean sponge, Calyx cf. Podatypa. J. Nat. Prod. 1995, 58, 201–208.
- Gautschi, M.; Schmid, J.P.; Peppard, T.L.; Ryan, T.P.; Tuorto, R.M.; Yang, X. Chemical characterization of diketopiperazines in beer. J. Agric. Food Chem. 1997, 45, 3183–3189.
- Stark, T.; Hofmann, T. Structures, sensory activity, and dose/response functions of 2,5-diketopiperazines in roasted cocoa nibs (Theobroma cacao). J. Agric. Food Chem. 2005, 53, 7222–7231.
- Fdhila, F.; Vázquez, V.; Sánchez, J.L.; Riguera, R. DD-diketopiperazines: Antibiotics active against Vibrio anguillarum isolated from marine bacteria associated with cultures of Pecten maximus. J. Nat. Prod. 2003, 66, 1299–1301.
- Kim, C.K.; Woo, J.K.; Lee, Y.J.; Lee, H.S.; Sim, C.J.; Oh, D.C.; Oh, K.B.; Shin, J. Callyazepin and (3R)-methylazacyclodecane, nitrogenous macrocycles from a Callyspongia sp. sponge. J. Nat. Prod. 2016, 79, 1179–1183.
- López, S.; Fernández-Trillo, F.; Midón, P.; Castedo, L.; Saá, C. First stereoselective syntheses of (−)-siphonodiol and (−)-tetrahydrosiphonodiol, bioactive polyacetylenes from marine sponges. J. Org. Chem. 2005, 70, 6346–6352.
- Díaz, Y.M.; Laverde, G.V.; Gamba, L.R.; Wandurraga, H.M.; Arévalo-Ferro, C.; Rodríguez, F.R.; Beltrán, C.D.; Hernández, L.C. Biofilm inhibition activity of compounds isolated from two Eunicea species collected at the Caribbean Sea. Rev. Bras. Farmacogn. 2015, 25, 605–611.
- Shi, Z.; Jain, S.; Kim, I.W.; Peng, X.X.; Abraham, I.; Youssef, D.T.A.; Fu, L.W.; Sayed, K.; Ambudkar, S.V.; Chen, Z.S. Sipholenol A, a marine-derived sipholane triterpene, potently reverses P-glycoprotein (ABCB1)-mediated multidrug resistance in cancer cells. Cancer Sci. 2007, 98, 1373–1380.
- Ma, J.; Fu, G.; Wu, J.; Han, S.; Zhang, L.; Yang, M.; Yu, Y.; Zhang, M.; Lin, Y.; Wang, Y. 4-cholesten-3-one suppresses lung adenocarcinoma metastasis by regulating translocation of HMGB1, HIF1α and Caveolin-1. Cell Death Dis. 2016, 7, e2372.
- Villaseñor, I.M.; Angelada, J.; Canlas, A.P.; Echegoyen, D. Bioactivity studies on β-sitosterol and its glucoside. Phytother. Res. 2002, 16, 417–421.
- Choi, S.; Kim, K.W.; Choi, J.S.; Han, S.T.; Park, Y.I.; Lee, S.K.; Kim, J.S.; Chung, M.H. Angiogenic activity of β-sitosterol in the ischaemia/reperfusion-damaged brain of mongolian gerbil. Planta Med. 2002, 68, 330–335.
- Beltrame, F.L.; Pessini, G.L.; Doro, D.L.; Filho, B.P.D.; Bazotte, R.B.; Cortez, D.A.G. Evaluation of the antidiabetic and antibacterial activity of Cissus sicyoides. Braz. Arch. Biol. Technol. 2002, 45, 21–25.
- Sen, A.; Dhavan, P.; Shukla, K.K.; Singh, S.; Tejovathi, G. Analysis of IR, NMR and antimicrobial activity of β-sitosterol isolated from Momordica charantia. Sci. Secur. J. Biotech. 2012, 1, 9–13.
- Kiprono, P.C.; Kaberia, F.; Keriko, J.M.; Karanja, J.N. The in vitro Anti-fungal and anti-bacterial activities of β-sitosterol from Senecio lyratus (Asteraceae). Verl. Z. Nat. 2000, 55, 485–488.
- Zeb, M.A.; Khan, S.U.; Rahman, T.U.; Sajid, M.; Seloni, S. Isolation and biological activity of β-sitosterol and stigmasterol from the roots of Indigofera heterantha. Pharm. Pharmacol. Int. J. 2017, 5, 204–207.
- Nirmal, S.A.; Pal, S.C.; Mandal, S.C.; Patil, A.N. Analgesic and anti-inflammatory activity of β-sitosterol isolated from Nyctanthes arbortristis leaves. Inflammopharmacology 2012, 20, 219–224.
- Gupta, M.B.; Nath, R.; Srivastava, N.; Shanker, K.; Kishor, K.; Bhargava, K.P. Anti-inflammatory and antipyretic activities of β-sitosterol. Planta Med. 1980, 39, 157–163.
- Loizou, S.; Lekakis, I.; Chrousos, G.P.; Moutsatsou, P. β-Sitosterol exhibits anti-inflammatory activity in human aortic endothelial cells. Mol. Nutr. Food Res. 2010, 54, 551–558.
- Chai, J.W.; Kuppusamy, U.R.; Kanthimathi, M.S. Beta-sitosterol induces apoptosis in MCF-7 Cells. Malays. J. Biochem. Mol. Biol. 2008, 16, 28–30.
- Awad, A.B.; Holtz, R.L.; Cone, J.P.; Fink, C.S.; Chen, Y.C. Beta-sitosterol inhibits growth of HT-29 human colon cancer cells by activating the sphingomyelin cycle. Anticancer Res. 1998, 18, 471–473.
- Park, C.; Moon, D.O.; Rhu, C.H.; Choi, B.T.; Lee, W.H.; Kim, G.Y.; Choi, Y.H. β-sitosterol induces anti-proliferation and apoptosis in human leukemic U937 cells through activation of caspase-3 and induction of Bax/Bcl-2 ratio. Biol. Pharm. Bull. 2007, 30, 1317–1323.
- Vundru, S.S.; Kale, R.K.; Singh, R.P. β-sitosterol induces G1 arrest and causes depolarization of mitochondrial membrane potential in breast carcinoma MDA-MB-231 cells. BMC Complement. Altern. Med. 2013, 13, 280.
- Zhao, Y.; Chang, S.K.C.; Qu, G.; Li, T.; Cui, H. β-sitosterol inhibits cell growth and induces apoptosis in SGC-7901 human stomach cancer cells. J. Agric. Food Chem. 2009, 57, 5211–5218.
- Holtz, R.L.; Fink, C.S.; Awad, A.B. β-sitosterol activates the sphingomyelin cycle and induces apoptosis in LNCaP human prostate cancer cells. Nutr. Cancer 1998, 32, 8–12.
- Sugano, M.; Morioka, H.; Ikeda, I. A Comparison of hypocholesterolemic activity of β-sitosterol and β-sitostanol in rats. J. Nutr. 1977, 107, 2011–2019.
- Fraile, L.; Crisci, E.; Córdoba, L.; Navarro, M.A.; Osada, J.; Montoya, M. Immunomodulatory properties of beta-sitosterol in pig immune responses. Int. Immunopharmacol. 2012, 13, 316–321.




