
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jakub Litak | + 1020 word(s) | 1020 | 2021-10-20 05:28:14 | | | |
| 2 | Catherine Yang | Meta information modification | 1020 | 2021-12-15 08:52:01 | | |
Video Upload Options
Glioblastoma (GBM) is the most popular primary central nervous system cancer and has an extremely expansive course. Aggressive tumor growth correlates with short median overall survival (OS) oscillating between 14 and 17 months. The survival rate of patients in a three-year follow up oscillates around 10%. The interaction of the proteins programmed death-1 (PD-1) and programmed cell death ligand (PD-L1) creates an immunoregulatory axis promoting invasion of glioblastoma multiforme cells in the brain tissue. The PD-1 pathway maintains immunological homeostasis and protects against autoimmunity. PD-L1 expression on glioblastoma surface promotes PD-1 receptor activation in microglia, resulting in the negative regulation of T cell responses.
1. Introduction
2. PD-L1 and PD-1 Structure
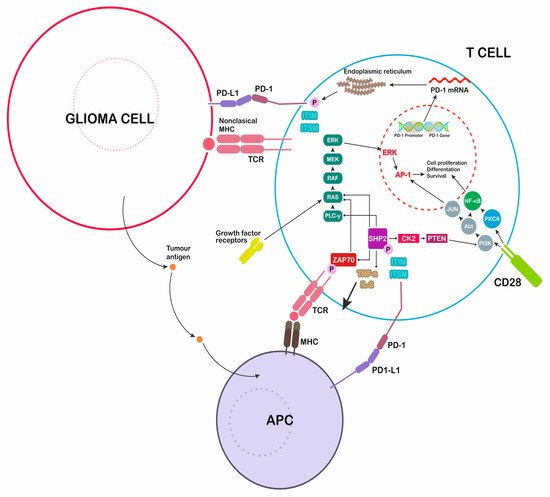
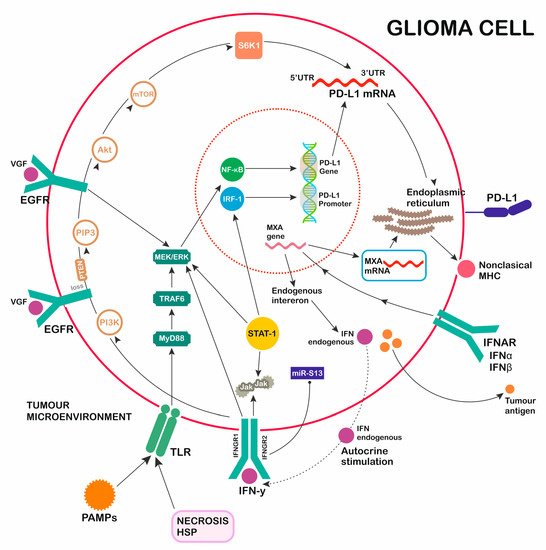
3. PD-1 Ligand Expression—Role of TLR Activation
References
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 3–9.
- Zarnett, O.J.; Sahgal, A.; Gosio, J.; Perry, J.; Berger, M.S.; Chang, S.; Das, S. Treatment of elderly patients with glioblastoma: A systematic evidence-based analysis. JAMA Neurol. 2015, 72, 589–596.
- Greer, L.; Pannullo, S.C.; Smith, A.W.; Taube, S.; Yondorf, M.Z.; Parashar, B.; Trichter, S.; Nedialkova, L.; Sabbas, A.; Christos, P.; et al. Accelerated hypofractionated radiotherapy in the era of concurrent temozolomide chemotherapy in elderly patients with glioblastoma multiforme. Cureus 2017, 9, e1388.
- Fernandes, C.; Costa, A.; Osório, L.; Lago, R.C.; Linhares, P.; Carvalho, B.; Caeiro, C. Current standards of care in glioblastoma therapy. In Glioblastoma ; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, Australia, 2007; Chapter 11.
- Roy, S.; Lahiri, D.; Maji, T.; Biswas, J. Recurrent glioblastoma: Where we stand. South Asian J. Cancer 2015, 4, 163–173.
- Phillips, H.S.; Kharbanda, S.; Chen, R.; Forrest, W.F.; Soriano, R.H.; Wu, T.D.; Misra, A.; Nigro, J.M.; Colman, H.; Soroceanu, L.; et al. Molecularn subclasses of high-grade glioma predict prognosis, delineatea pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006, 9, 157–173.
- Zeng, J.; See, A.P.; Phallen, J.; Jackson, C.M.; Belcaid, Z.; Ruzevick, J.; Durham, N.; Meyer, C.; Harris, T.J.; Albesiano, E.; et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. 2013, 86, 343–349.
- Freeman, G.J.; Hochrein, H.; O’Keeffe, M.; Luft, T.; Vandenabeele, S.; Grumont, R.J.; Maraskovsky, E.; Shortman, K. Engagement of the PD-1 immunoinhibitory receptor by a novel b7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034.
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999, 5, 1365–1369.
- Zhang, X.; Schwartz, J.C.; Guo, X.; Bhatia, S.; Cao, E.; Chen, L.; Zhang, Z.Y.; Edidin, M.A.; Nathenson, S.G.; Almo, S.C. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity 2004, 20, 337–347.
- Cheng, X.; Veverka, V.; Radhakrishnan, A.; Waters, L.C.; Muskett, F.W.; Morgan, S.H.; Huo, J.; Yu, C.; Evans, E.J.; Leslie, A.J.; et al. Structure and interactions of the human programmed cell death 1 receptor. J. Biol. Chem. 2013, 288, 11771–11785.
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895.
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 311–319.
- Baruch, K.; Deczkowska, A.; Rosenzweig, N.; Tsitsou-Kampeli, A.; Sharif, A.M.; Matcovitch-Natan, O.; Kertser, A.; David, E.; Amit, I.; Schwartz, M. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat. Med. 2016, 22, 135–137.
- Yokosuka, T.; Takamatsu, M.; Kobayashi-Imanishi, W.; Hashimoto-Tane, A.; Azuma, M.; Saito, T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J. Exp. Med. 2012, 209, 1201–1217.
- Kawai, T.; Akira, S. TLR signaling. Cell Death Differ. 2006, 13, 816–825.
- Asea, A. Novel signal transduction pathway utilized by extracellular hsprole of toll-like receptor (TLR) 2 and TLR4. J. Boil. Chem. 2002, 277, 15028–15034.
- Verstrepen, L.; Bekaert, T.; Chau, T.-L.; Tavernier, J.; Chariot, A.; Beyaert, R. TLR-4, IL-1R and TNF-R signaling to NF-κB: Variations on a common theme. Cell. Mol. Life Sci. 2008, 65, 2964–2978.
- Meng, Y.; Kujas, M.; Marie, Y.; Paris, S.; Thillet, J.; Delattre, J.-Y.; Carpentier, A.F. Expression of TLR9 within human glioblastoma. J. Neuro-Oncol. 2008, 88, 19–25.
- Medvedev, A.E.; Sabroe, I.; Hasday, J.D.; Vogel, S.N. Invited review: Tolerance to microbial TLR ligands: Molecular mechanisms and relevance to disease. J. Endotoxin Res. 2006, 12, 133–150.
- Aalaei-Andabili, S.H.; Rezaei, N. Toll like receptor (TLR)-induced differential expression of microRNAs (MiRs) and immune response against infection: A systematic review. J. Infect. 2013, 67, 251–264.
- Ampie, L.; Choy, W.; Lamano, J.B.; Fakurnejad, S.; Bloch, O.; Parsa, A.T. Heat shock protein vaccines against glioblastoma: From bench to bedside. J. Neuro-Oncol. 2015, 123, 441–448.
- Waziri, A. Glioblastoma-Derived Mechanisms of Systemic Immunosuppression. Neurosurg. Clin. N. Am. 2010, 21, 31–42.
- Ampie, L.; Woolf, E.C.; Dardis, C. Immunotherapeutic Advancements for Glioblastoma. Front. Oncol. 2015, 5, 12.
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511.
- O’Neill, L.A.; Bowie, A.G. The family of five: TIR-domaincontaining adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007, 7, 353.
- Echigo, R.; Sugimoto, N.; Yachie, A.; OhnoShosaku, T. Cannabinoids inhibit peptidoglycan-induced phosphorylation of NF-κB and cell growth in U87MG human malignant glioma cells. Oncol. Rep. 2012, 28, 1176–1180.




