Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | David Waisman | + 2753 word(s) | 2753 | 2021-12-14 07:17:56 | | | |
| 2 | Catherine Yang | Meta information modification | 2753 | 2021-12-15 02:23:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Waisman, D. The Annexin A2/S100A10 Complex. Encyclopedia. Available online: https://encyclopedia.pub/entry/17114 (accessed on 11 January 2026).
Waisman D. The Annexin A2/S100A10 Complex. Encyclopedia. Available at: https://encyclopedia.pub/entry/17114. Accessed January 11, 2026.
Waisman, David. "The Annexin A2/S100A10 Complex" Encyclopedia, https://encyclopedia.pub/entry/17114 (accessed January 11, 2026).
Waisman, D. (2021, December 14). The Annexin A2/S100A10 Complex. In Encyclopedia. https://encyclopedia.pub/entry/17114
Waisman, David. "The Annexin A2/S100A10 Complex." Encyclopedia. Web. 14 December, 2021.
Copy Citation
Mutualistic symbiosis refers to the symbiotic relationship between individuals of different species in which both individuals benefit from the association. S100A10, a member of the S100 family of Ca2+-binding proteins, exists as a tight dimer and binds two annexin A2 molecules. This association forms the annexin A2/S100A10 complex known as AIIt, and modifies the distinct functions of both proteins. Annexin A2 is a Ca2+-binding protein that binds F-actin, phospholipid, RNA, and specific polysaccharides such as heparin.
S100A10
annexin A2
plasminogen
plasmin
symbiosis
1. Introduction
The nomenclature for the S100 family of Ca2+-binding proteins is derived from observing that these proteins are soluble in a saturated ammonium sulfate solution at a neutral pH. The S100 family of proteins consists of about twenty 10–12 kDa, Ca2+-binding proteins that are expressed only in vertebrates and consist of S100A1–S100A18, S100B, S100G, S100P, S100Z, as well as members with an N-terminal S100 domain fused to additional C-terminal sequences, such as repetin [1], trichohyalin and filaggrin [2][3][4]. Of the twenty-five human S100 genes, 19 are located within chromosome 1q21. Other members (S100A11, S100B, S100G, S100P, and S100Z) map to different chromosome regions [5]. Each S100 protein has a unique expression and distribution profile amongst different tissues and cell types. The S100 proteins exist as anti-parallel homo- and heterodimers, with each monomer consisting of two helix-loop-helix EF-hand motif types, Ca2+-binding domains (EF-1 and EF-2) connected by a hinge region. The nomenclature for the EF-hand domain originally comes from the EF-hand motif first identified in the Ca2+-binding protein, parvalbumin, which is composed of two alpha-helices, “E” and “F” connected by an intermediate loop of 12 residues binding Ca2+ [6]. Specifically, the S100 proteins possess an S100-specific EF-hand at the N-terminus and a canonical EF-hand at the C-terminus. In all S100 proteins except S100A10, the EF-hands bind Ca2+. These EF-hands are not identical; the carboxyl-terminal EF-hand Ca2+-binding loop contains the classical 12-amino-acid long Ca2+-binding motif common to all EF-hand Ca2+-binding proteins, such as parvalbumin and calmodulin. In this motif, Ca2+-binding occurs via acidic side chains that comprise the sequence DXDGDGTIXXXE. However, the N-terminal EF-hand motif is a 14-amino-acid long Ca2+-binding loop that is characteristic of all S100 proteins and is referred to as the S100-specific or pseudo-EF domain. This motif binds Ca2+ via backbone carbonyl groups and one carboxylate side group contributed by glutamic acid (reviewed in [2][7][8]). All S100 proteins bind calcium, except S100A10, which has lost its ability to bind calcium due to substitutions in its calcium-binding loop but retains the “active” conformation of a calcium-bound S100 protein [9]. S100A10 lost its ability to bind calcium due to substitutions in its calcium-binding loop but retains the activated structure of a calcium-bound S100 protein [10] (Figure 1).
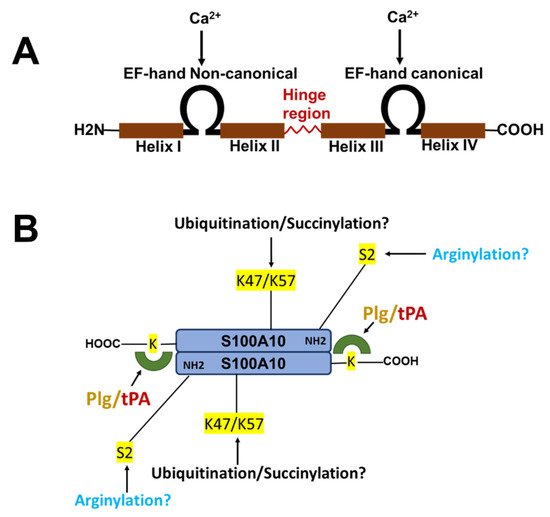
Figure 1. Structure of S100 proteins. (A) The structure of a typical S100 protein. (B) The structure and key regulatory sites of S100A10. Shown are the sites for ubiquitylation, arginylation, and succinylation.
The S100 proteins S100A1, S100A4, S100A6, S100A10, S100A11, S100A12, and S100B can interact with several annexin (ANX) proteins. The annexins are a family of proteins that bind multiple Ca2+ ions via Ca2+-binding sites that are not EF-hand motifs but are referred to as type II and type III Ca2+-binding sites [11]. The X-ray crystallographic analysis of ANXA2 established a single type II Ca2+-binding site in each of the second, third, and fourth domains and two type III Ca2+-binding sites in the first domain of the protein. The characteristic feature of all annexins is the presence of several copies of an annexin repeat, which is defined as an approximately 70-residue-long conserved structural element that is required for the Ca2+ and membrane-binding function of the annexins. The presence of such annexin repeats combined with the capability of a protein to interact with the negatively charged phospholipids in a Ca2+-dependent manner is the major criterion defining the family of annexins. There are 12 human annexins (ANXA1-ANXA11 and ANXA13). ANXA1, ANXA2, ANXA5, ANXA6, and ANXA11 interact with S100 proteins [12]. S100A1, S100A4, S100A6, S100A10, S100A11, S100A12, and S100B can interact with annexins. Interestingly, the S100 proteins and annexins can form multiple complexes. For example, ANXA2 is found in complexes with S100A4, S100A6, S100A10, and S100A11 [13]. However, the complex formation is much more favorable in the case of S100A10 (Kd of 13 nM) compared to S100A4 (Kd of 5 uM) [14]. Furthermore, although both S100A4 and S100A10 promote plasminogen activation [15][16], plasminogen activation by S100A4 but not S100A10 is Ca2+-dependent.
2. Mutualistic Symbiosis
Mutualistic symbiosis refers to the symbiotic relationship between individuals of different species in which both individuals benefit from the association. We propose that this biological relationship between species can be extended to the subunits within a protein complex. As will be discussed in detail, the formation of the ANXA2/S100A10 complex regulates the biological activity of each subunit and confers new biological functions that were not possessed by either subunit. Furthermore, complex formation is also critical for the survival of S100A10 because, in the absence of ANXA2, the protein is rapidly degraded [17].
2.1. Mutualistic Symbiosis I-Survival of S100A10
S100A10 is undetectable unless ANXA2 is also expressed [17][18][19][20][21]. Thus, S100A10 is an extrinsically disordered protein that is rapidly degraded [22]. However, the binding of S100A10 to DLC1 does not protect S100A10 from degradation [23]. However, ANXA2 competes with DLC1 for binding to S100A10, suggesting that the interaction of S100A10 with ANXA2 or DLC1 results in unique conformations of S100A10. Previous studies demonstrated that the depletion of ANXA2 from cells resulted in the rapid ubiquitylation and proteasomal degradation of S100A10. One of these studies utilized protein overexpression with site-directed S100A10 mutants to propose that the carboxyl-terminal residues, Lys-91 and/or Lys-93, function as the ubiquitylation sites [24]. However, these studies failed to directly confirm the presence of ubiquitylated S100A10 at these sites by mass spectrometric analysis. We have also observed that the forced expression of S100A10 and ubiquitin results in the ubiquitination of S100A10, and we identified, for the first time, that the ubiquitination of S100A10 in forced expression systems occurs on Lys-57 [25]. Our study also showed that the mutagenesis of Lys-57 prevents the ubiquitylation and degradation of overexpressed S100A10. In contrast, another group identified Lys-47 as a site of the succinylation of S100A10 and proposed that this site was also a site of ubiquitylation [26]. Interestingly, Lys-47, Lys-54, and Lys-57 were identified as sites of the ubiquitylation of S100A10 by the mass spectrometric analysis of tissue extracts [27], although these sites were not confirmed by site-directed mutagenesis. It was also not clear why only two of the five tissues examined in that study detected ubiquitinated S100A10. It is interesting to note that intrinsically disordered regions have been identified in S100A10 and that Lys-57 is located in the helix III, which is an intrinsically disordered region [22]. We analyzed the amino acid sequence of the S100A10 of twenty-one vertebrate species. Although Lys-57 is conserved in all vertebrate species, Lys-47 is not conserved in chickens, Xenopus or pufferfish.
2.2. Mutualistic Symbiosis II-Regulation of the Biological Functions of ANXA2 and S100A10
The mutualistic symbiotic role of S100A10 binding is apparent from studies of the regulation of F-actin bundling. Individually, S100A10 and ANXA2 are incapable of the significant bundling of F-actin; however, when ANXA2 bound S100A10, the complex facilitated the Ca2+-dependent formation of anisotropic F-actin bundles [28]. Because ANXA2 is an F-actin-binding protein, whereas S100A10 does not interact with F-actin, this type of mutualistic symbiosis involves modifying the function of one subunit by the other.
AIIt-dependent F-actin bundles participate in the spatial organization of the plasma membrane, providing active sites for secretory granule docking and exocytotic fusion. For example, it has been shown that when chromaffin cells are stimulated with nicotine, the F-actin-bundling activity of ANXA2 promotes the formation of the monosialotetrahexosylganglioside and GM1-enriched microdomains at the plasma membrane, increases the number of morphologically docked granules at the plasma membrane, and controls the number of individual exocytotic events [29]. Conversely, when an ANXA2 mutant with impaired F-actin filament–bundling activity [30] was expressed, the formation of plasma membrane lipid microdomains and the number of exocytotic events were decreased, and the fusion kinetics were slower [29]. Based on these studies, the authors concluded that AIIt-induced F-actin bundling is essential for generating active exocytotic sites.
Chasserot-Golaz’s group proposed a model for the involvement of AIIt in neurosecretion that incorporates both the F-actin bundling and the lipid sequestration properties of AIIt [31]. In unstimulated adrenergic cells, ANXA2 is cytosolic, S100A10 is bound to vesicle-associated membrane protein 2 (VAMP2) at the plasma membrane, and the membrane lipids PIP2 and GM1 are randomly distributed in the plasma membrane. However, upon stimulation with secretagogues, ANXA2 translocates to the plasma membrane in proximity to N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes; this redistribution of ANXA2 is mediated by S100A10 [32]. The newly formed AIIt forms specific lipid microdomains. AIIt then bundles F-actin, which constrains the lateral mobility of the newly formed lipid domains, thereby increasing their stability. This compartmentalization of the plasma membrane serves to spatially and temporally organize F-actin bundles, which contribute to the docking machinery and thereby accelerate the vesicle fusion proteins required for secretory granule docking and fusion, likely playing a role in both the speed and accuracy of the exocytotic fusion process.
The biochemical properties of the monomeric ANXA2 and ANXA2 present in the AIIt complex are distinct. ANXA2 typically exists as a monomer predominantly localized to the cytosol and on early endosomes [33] or as a heterotetrameric complex—AIIt—found in the subplasmalemmal region [34]. Several laboratories have reported that the binding of S100A10 to ANXA2 induces a conformational change in ANXA2, which influences its interaction with several of its ligands. For example, S100A10 binding has a stimulatory effect on phospholipid binding by ANXA2, and the phospholipid requirement for 50% binding is 10-fold lower for the complex than ANXA2 [35]. We initially investigated the consequence of complex formation on the biological properties of the individual subunits by expressing and purifying recombinant ANXA2 and S100A10 and then combining the subunits and isolating AIIt. Previously, it was reported that only the ANXA2/S100A10 complex—and not monomeric ANXA2—could aggregate membrane vesicles at submicromolar Ca2+ concentrations [36][37]. We found that the binding of recombinant S100A10 to recombinant ANXA2 resulted in a decrease in the A0.5 (Ca2+) for chromaffin granule aggregation from 0.23 mM for recombinant ANXA2 to 1.0 μM for the recombinant complex. We also found that the binding of recombinant S100A10 to recombinant ANXA2 resulted in a decrease in the A0.5 (Ca2+) of phospholipid liposome aggregation from 0.83 μM for recombinant ANXA2 to 0.26 μM for the recombinant complex. In contrast, S100A10 did not interact with chromaffin granules or phospholipid vesicles.
The thioredoxin system reduces AIIt, enabling it to repeatedly exert its protein reductase activity. Because the mutagenesis of ANXA2 Cys-334-Ser and either S100A10 Cys-61-Ser or S100A10 Cys-82-Ser inactivated the plasmin reductase activity of the isolated subunits of AIIt, and because the sum of the plasmin reactivity (AIIt) is greater than the activity exhibited by each subunit, we concluded that each subunit acts in a mutualistic symbiotic relationship to enhance its plasmin reductase activity.
2.3. Mutualistic Symbiosis III—Acquisition of New Biological Activities by the Formation of the ANXA2/S100A10 Complex
The complex formed between ANXA2, S100A10, and AHNAK also interact with dysferlin [38] (Figure 2). This large multiprotein complex facilitates the wound repair of damaged epithelial, auditory, and muscle cells upon extracellular calcium influx [39][40][41][42]. Mechanistically, it has been suggested that this dysferlin membrane repair complex, when activated by calcium, promotes the fusion of exocytosis vesicles with the inner side of the plasma membrane, resulting in the resealing of membrane tears [43][44]. The ANXA2-mediated linking of membrane surfaces under non-oxidative intracellular conditions requires ANXA2-S100A10 complex formation because ANXA2 by itself cannot link biological membranes [37]. This suggests that the formation of the AIIt complex and its mutualistic symbiosis is required for membrane repair and that individually ANXA2 or S100A10 cannot participate in this process.
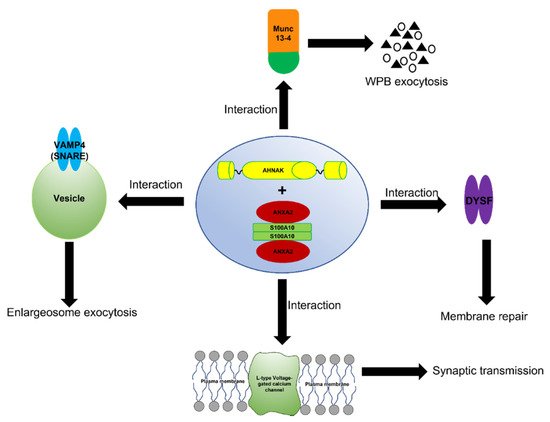
Figure 2. Schematic representation of the function of the AIIt-AHNAK complex. The formation of the ANXA2/S100A10/AHNAK (2:2:1) complex forms a key hub for the regulation of several important biological functions such as the exocytosis of enlargesomes, the regulation of the L-type voltage-gated calcium channel (VGCC), and possibly the MUNC-13-dependent regulation of the exocytosis of Weibel–Palade bodies (WPB).
The ANXA2/S100A10/AHNAK complex forms a complex with the L-type voltage-gated calcium channel (VGCC) [45], which plays a role in regulating Ca2+-dependent exocytosis. These channels are the primary regulators that control Ca2+ release and are pharmacological targets for treating cardiac ischemia and hypertension [46]. A decrease in the L-type calcium influx was observed in both the glutamatergic neurons and GABAergic interneurons of AHNAK-KO mice, suggesting that L-type calcium channels may act as effectors of the ANXA2/S100A10/AHNAK complex.
Recently, S100A10 was demonstrated to interact with the background potassium channel TASK-1 and regulate the membrane trafficking and the functionality of this channel (Girard et al., 2002). The tetrodotoxin-insensitive voltage-gated sodium channel, Nav1.8, has also been shown to bind S100A10 (Okuse et al., 2002). S100A10 promoted the translocation of Nav1.8 to the plasma membrane, thereby producing functional sodium channels. The role of ANXA2 has not been assessed for TASK-1 or Nav1.8. Therefore, it is unclear whether both ANXA2 and S100A10 are required for the activity of these ion channels.
Metabotropic glutamate receptors (mGluRs) are a sub-family of glutamate receptors. Previous studies have shown that antagonists acting on mGluR5 or mGluR2/3 exert antidepressant-like activities in mice [47]. Interestingly, an S100A10-binding motif has been identified in the cytoplasmic tail of mGluR5, and a C83Q S100A10 mutant which cannot interact with ANXA2, failed to interact with mGluR5. Therefore, the authors concluded that ANXA2 is required for the interaction of S100A10 with mGluR5 [48]. Accordingly, ANXA2 and S100A10 act in a mutualistic symbiotic relationship resulting in the regulation of mGluR5.
Mutations in CFTR, a cAMP/PKA, and the ATP-regulated Cl- channel result in the disease cystic fibrosis. AIIt forms a PKA/calcineurin-dependent complex with CFTR and tethers the complex to the plasma membrane [49]. Because the forced disruption of AIIt by a peptide to the S100A10 binding site disrupts the AIIt-CFTR complex and inhibits outwardly rectifying Cl− channels, the authors concluded that both subunits of AIIt were required for channel activity. Therefore, ANXA2 and S100A10 act in a mutualistic symbiotic relationship resulting in the regulation of CFTR.
Endosomes are subcellular organelles associated with the catabolism of exogenous and endogenous proteins and the down-regulation of surface receptors. They exist as three distinct cellular compartments: early endosomes, late endosomes, and recycling endosomes. S100A10 has been suggested to be dispensable for ANXA2 functions in transporting endocytosed tracers from early to late endosomes. The N-terminus of ANXA2 has a membrane-binding motif (amino acids 15 to 24) that regulates its Ca2+-independent association with endosomes [50]. The depletion of ANXA2 prevents newly-formed multivesicular endosomes from detaching from early endosomes and regulates their transport towards late endosomes [33]. However, AIIt plays a role in the subcellular distribution of early and recycling endosomes [21][51]. To account for these differences, it has been suggested that calcium-dependent membrane association at the plasma membrane or along the protein recycling pathway is regulated by S100A10 binding and AIIt formation [52]. For example, S100A10 appears necessary for ANXA2 binding to the plasma membrane and the cortical actin cytoskeleton. Utilizing HPV as model cargo and BSA as pathway-specific canonical cargo in uptake experiments, Taylor et al. [53] demonstrated that S100A10 and ANXA2 are required for endosomal vesicular trafficking. They also suggested that AIIt functions in the biogenesis of multivesicular endosomes. Therefore, ANXA2 and S100A10 act in a mutualistic symbiotic relationship to regulate certain aspects of the endosomal pathway.
In conclusion, ANXA2 and S100A10 collaborate in regulating multiple cellular functions, including specific ion channels. In some cases, S100A10 and ANXA2 provide binding sites for an ion channel, and in other cases, each subunit may play distinct but essential regulatory roles. In some instances, ANXA2 may tether the S100A10-ligand/channel complex to the plasma membrane. The ANXA2-S100A10-AHNAK complex also plays key roles in regulating L-type voltage-gated calcium channels and in the membrane repair process.
References
- Krieg, P.; Schuppler, M.; Koesters, R.; Mincheva, A.; Lichter, P.; Marks, F. Repetin (Rptn), a New Member of the “Fused Gene” Subgroup within the S100 Gene Family Encoding a Murine Epidermal Differentiation Protein. Genomics 1997, 43, 339–348.
- Donato, R. S100: A Multigenic Family of Calcium-Modulated Proteins of the EF-Hand Type with Intracellular and Extracellular Functional Roles. INTJ Biochem. Biol. 2001, 33, 637–668.
- Gonzalez, L.L.; Garrie, K.; Turner, M.D. Role of S100 Proteins in Health and Disease. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118677.
- Mischke, D.; Korge, B.P.; Marenholz, I.; Volz, A.; Ziegler, A. Genes Encoding Structural Proteins of Epidermal Cornification and S100 Calcium-Binding Proteins Form a Gene Complex (“epidermal Differentiation Complex”) on Human Chromosome 1q21. J. Investig. Dermatol. 1996, 106, 989–992.
- Marenholz, I.; Heizmann, C.W.; Fritz, G. S100 Proteins in Mouse and Man: From Evolution to Function and Pathology (Including an Update of the Nomenclature). Biochem. Biophys. Res. Commun. 2004, 322, 1111–1122.
- Kretsinger, R.H.; Nockolds, C.E. Carp Muscle Calcium-Binding Protein II. Structure determination and general description. J. Biol. Chem. 1973, 248, 3313–3326.
- Allgöwer, C.; Kretz, A.-L.; von Karstedt, S.; Wittau, M.; Henne-Bruns, D.; Lemke, J. Friend or Foe: S100 Proteins in Cancer. Cancers 2020, 12, 2037.
- Madureira, P.A.; O’Connell, P.A.; Surette, A.P.; Miller, V.A.; Waisman, D.M. The Biochemistry and Regulation of S100A10: A Multifunctional Plasminogen Receptor Involved in Oncogenesis. J. Biomed. Biotechnol. 2012, 2012, 353687.
- Gerke, V.; Weber, K. The Regulatory Chain in the P36-Kd Substrate Complex of Viral Tyrosine-Specific Protein Kinases Is Related in Sequence to the S-100 Protein of Glial Cells. EMBO J. 1985, 4, 2917–2920.
- Gerke, V.; Weber, K. Calcium-Dependent Conformational Changes in the 36-KDa Subunit of Intestinal Protein I Related to the Cellular 36-KDa Target of Rous Sarcoma Virus Tyrosine Kinase. J. Biol. Chem. 1985, 260, 1688–1695.
- Weng, X.; Luecke, H.; Song, I.S.; Kang, D.S.; Kim, S.H.; Huber, R. Crystal Structure of Human Annexin I at 2.5 A Resolution. Protein Sci. Publ. Protein Soc. 1993, 2, 448–458.
- Gerke, V.; Moss, S.E. Annexins: From Structure to Function. Physiol. Rev. 2002, 82, 331–371.
- Miwa, N.; Uebi, T.; Kawamura, S. S100-Annexin Complexes—Biology of Conditional Association. FEBS J. 2008, 275, 4945–4955.
- Ecsédi, P.; Kiss, B.; Gógl, G.; Radnai, L.; Buday, L.; Koprivanacz, K.; Liliom, K.; Leveles, I.; Vértessy, B.; Jeszenői, N.; et al. Regulation of the Equilibrium between Closed and Open Conformations of Annexin A2 by N-Terminal Phosphorylation and S100A4-Binding. Structure 2017, 25, 1195–1207.e5.
- Semov, A.; Moreno, M.J.; Onichtchenko, A.; Abulrob, A.; Ball, M.; Ekiel, I.; Pietrzynski, G.; Stanimirovic, D.; Alakhov, V. Metastasis-Associated Protein S100A4 Induces Angiogenesis through Interaction with Annexin II and Accelerated Plasmin Formation. J. Biol. Chem. 2005, 280, 20833–20841.
- Kassam, G.; Le, B.H.; Choi, K.S.; Kang, H.M.; Fitzpatrick, S.L.; Louie, P.; Waisman, D.M. The P11 Subunit of the Annexin II Tetramer Plays a Key Role in the Stimulation of T-PA-Dependent Plasminogen Activation. Biochemistry 1998, 37, 16958–16966.
- Puisieux, A.; Ji, J.; Ozturk, M. Annexin II Up-Regulates Cellular Levels of P11 Protein by a Post-Translational Mechanisms. Biochem. J. 1996, 313 Pt 1, 51–55.
- Brandherm, I.; Disse, J.; Zeuschner, D.; Gerke, V. CAMP-Induced Secretion of Endothelial von Willebrand Factor Is Regulated by a Phosphorylation/Dephosphorylation Switch in Annexin A2. Blood 2013, 122, 1042–1051.
- Hou, Y.; Yang, L.; Mou, M.; Hou, Y.; Zhang, A.; Pan, N.; Qiang, R.; Wei, L.; Zhang, N. Annexin A2 Regulates the Levels of Plasmin, S100A10 and Fascin in L5178Y Cells. Cancer Investig. 2008, 26, 809–815.
- Zhang, J.; Guo, B.; Zhang, Y.; Cao, J.; Chen, T. Silencing of the Annexin II Gene Down-Regulates the Levels of S100A10, c-Myc, and Plasmin and Inhibits Breast Cancer Cell Proliferation and Invasion. Saudi Med. J. 2010, 31, 374–381.
- Zobiack, N.; Rescher, U.; Ludwig, C.; Zeuschner, D.; Gerke, V. The Annexin 2/S100A10 Complex Controls the Distribution of Transferrin Receptor-Containing Recycling Endosomes. Mol. Biol. Cell 2003, 14, 4896–4908.
- Permyakov, S.E.; Ismailov, R.G.; Xue, B.; Denesyuk, A.I.; Uversky, V.N.; Permyakov, E.A. Intrinsic Disorder in S100 Proteins. Mol. BioSyst. 2011, 7, 2164–2180.
- Yang, X.; Popescu, N.C.; Zimonjic, D.B. DLC1 Interaction with S100A10 Mediates Inhibition of in Vitro Cell Invasion and Tumorigenicity of Lung Cancer Cells through a RhoGAP-Independent Mechanism. Cancer Res. 2011, 71, 2916–2925.
- He, K.-L.; Deora, A.B.; Xiong, H.; Ling, Q.; Weksler, B.B.; Niesvizky, R.; Hajjar, K.A. Endothelial Cell Annexin A2 Regulates Polyubiquitination and Degradation of Its Binding Partner S100A10/P11. J. Biol. Chem. 2008, 283, 19192–19200.
- Holloway, R.W.; Thomas, M.L.; Cohen, A.M.; Bharadwaj, A.G.; Rahman, M.; Marcato, P.; Marignani, P.A.; Waisman, D.M. Regulation of Cell Surface Protease Receptor S100A10 by Retinoic Acid Therapy in Acute Promyelocytic Leukemia (APL). Cell Death Dis. 2018, 9, 920.
- Wang, C.; Zhang, C.; Li, X.; Shen, J.; Xu, Y.; Shi, H.; Mu, X.; Pan, J.; Zhao, T.; Li, M.; et al. CPT1A-Mediated Succinylation of S100A10 Increases Human Gastric Cancer Invasion. J. Cell. Mol. Med. 2019, 23, 293–305.
- Wagner, S.A.; Beli, P.; Weinert, B.T.; Schölz, C.; Kelstrup, C.D.; Young, C.; Nielsen, M.L.; Olsen, J.V.; Brakebusch, C.; Choudhary, C. Proteomic Analyses Reveal Divergent Ubiquitylation Site Patterns in Murine Tissues. Mol. Cell. Proteom. MCP 2012, 11, 1578–1585.
- Ikebuchi, N.W.; Waisman, D.M. Calcium-Dependent Regulation of Actin Filament Bundling by Lipocortin-85. J. Biol. Chem. 1990, 265, 3392–3400.
- Gabel, M.; Delavoie, F.; Demais, V.; Royer, C.; Bailly, Y.; Vitale, N.; Bader, M.-F.; Chasserot-Golaz, S. Annexin A2–Dependent Actin Bundling Promotes Secretory Granule Docking to the Plasma Membrane and Exocytosis. J. Cell Biol. 2015, 210, 785–800.
- Jones, P.G.; Moore, G.J.; Waisman, D.M. A Nonapeptide to the Putative F-Actin Binding Site of Annexin-II Tetramer Inhibits Its Calcium-Dependent Activation of Actin Filament Bundling. J. Biol. Chem. 1992, 267, 13993–13997.
- Gabel, M.; Chasserot-Golaz, S. Annexin A2, an Essential Partner of the Exocytotic Process in Chromaffin Cells. J. Neurochem. 2016, 137, 890–896.
- Umbrecht-Jenck, E.; Demais, V.; Calco, V.; Bailly, Y.; Bader, M.-F.; Chasserot-Golaz, S. S100A10-Mediated Translocation of Annexin-A2 to SNARE Proteins in Adrenergic Chromaffin Cells Undergoing Exocytosis. Traffic 2010, 11, 958–971.
- Morel, E.; Gruenberg, J. The P11/S100A10 Light Chain of Annexin A2 Is Dispensable for Annexin A2 Association to Endosomes and Functions in Endosomal Transport. PLoS ONE 2007, 2, e1118.
- Thiel, C.; Osborn, M.; Gerke, V. The Tight Association of the Tyrosine Kinase Substrate Annexin II with the Submembranous Cytoskeleton Depends on Intact P11- and Ca(2+)-Binding Sites. J. Cell Sci. 1992, 103, 733–742.
- Powell, M.A.; Glenney, J.R. Regulation of Calpactin I Phospholipid Binding by Calpactin I Light-Chain Binding and Phosphorylation by P60v-Src. Biochem. J. 1987, 247, 321–328.
- Drust, D.S.; Creutz, C.E. Aggregation of Chromaffin Granules by Calpactin at Micromolar Levels of Calcium. Nature 1988, 331, 88–91.
- Grill, D.; Matos, A.L.L.; de Vries, W.C.; Kudruk, S.; Heflik, M.; Dörner, W.; Mootz, H.D.; Jan Ravoo, B.; Galla, H.-J.; Gerke, V. Bridging of Membrane Surfaces by Annexin A2. Sci. Rep. 2018, 8, 14662.
- Huang, Y.; Laval, S.H.; Van Remoortere, A.; Baudier, J.; Benaud, C.; Anderson, L.V.B.; Straub, V.; Deelder, A.; Frants, R.R.; Den Dunnen, J.T.; et al. AHNAK, a Novel Component of the Dysferlin Protein Complex, Redistributes to the Cytoplasm with Dysferlin during Skeletal Muscle Regeneration. FASEB J. 2007, 21, 732–742.
- Rezvanpour, A.; Santamaria-Kisiel, L.; Shaw, G.S. The S100A10-Annexin A2 Complex Provides a Novel Asymmetric Platform for Membrane Repair. J. Biol. Chem. 2011, 286, 40174–40183.
- Yan, X.; Kumar, K.; Miclette Lamarche, R.; Youssef, H.; Shaw, G.S.; Marcotte, I.; DeWolf, C.E.; Warschawski, D.E.; Boisselier, E. Interactions between the Cell Membrane Repair Protein S100A10 and Phospholipid Monolayers and Bilayers. Langmuir 2021, 37, 9652–9663.
- Cacciottolo, M.; Belcastro, V.; Laval, S.; Bushby, K.; di Bernardo, D.; Nigro, V. Reverse Engineering Gene Network Identifies New Dysferlin-Interacting Proteins. J. Biol. Chem. 2011, 286, 5404–5413.
- de Morrée, A.; Hensbergen, P.J.; van Haagen, H.H.H.B.M.; Dragan, I.; Deelder, A.M.; ’t Hoen, P.A.C.; Frants, R.R.; van der Maarel, S.M. Proteomic Analysis of the Dysferlin Protein Complex Unveils Its Importance for Sarcolemmal Maintenance and Integrity. PLoS ONE 2010, 5, e13854.
- Draeger, A.; Monastyrskaya, K.; Babiychuk, E.B. Plasma Membrane Repair and Cellular Damage Control: The Annexin Survival Kit. Biochem. Pharmacol. 2011, 81, 703–712.
- Dempsey, B.R.; Rezvanpour, A.; Lee, T.-W.; Barber, K.R.; Junop, M.S.; Shaw, G.S. Structure of an Asymmetric Ternary Protein Complex Provides Insight for Membrane Interaction. Structure 2012, 20, 1737–1745.
- Jin, J.; Bhatti, D.L.; Lee, K.-W.; Medrihan, L.; Cheng, J.; Wei, J.; Zhong, P.; Yan, Z.; Kooiker, C.; Song, C.; et al. Ahnak Scaffolds P11/Anxa2 Complex and L-Type Voltage-Gated Calcium Channel and Modulates Depressive Behavior. Mol. Psychiatry 2020, 25, 1035–1049.
- Haase, H. Ahnak, a New Player in Beta-Adrenergic Regulation of the Cardiac L-Type Ca2+ Channel. Cardiovasc. Res. 2007, 73, 19–25.
- Pałucha-Poniewiera, A.; Wierońska, J.M.; Brański, P.; Burnat, G.; Chruścicka, B.; Pilc, A. Is the MGlu5 Receptor a Possible Target for New Antidepressant Drugs? Pharmacol. Rep. PR 2013, 65, 1506–1511.
- Lee, K.-W.; Westin, L.; Kim, J.; Chang, J.C.; Oh, Y.-S.; Amreen, B.; Gresack, J.; Flajolet, M.; Kim, D.; Aperia, A.; et al. Alteration by P11 of MGluR5 Localization Regulates Depression-like Behaviors. Mol. Psychiatry 2015, 20, 1546–1556.
- Borthwick, L.A.; McGaw, J.; Conner, G.; Taylor, C.J.; Gerke, V.; Mehta, A.; Robson, L.; Muimo, R. The Formation of the CAMP/Protein Kinase A-Dependent Annexin 2-S100A10 Complex with Cystic Fibrosis Conductance Regulator Protein (CFTR) Regulates CFTR Channel Function. Mol. Biol. Cell 2007, 18, 3388–3397.
- Jost, M.; Zeuschner, D.; Seemann, J.; Weber, K.; Gerke, V. Identification and Characterization of a Novel Type of Annexin-Membrane Interaction: Ca2+ Is Not Required for the Association of Annexin II with Early Endosomes. J. Cell Sci. 1997, 110 Pt 2, 221–228.
- Harder, T.; Gerke, V. The Subcellular Distribution of Early Endosomes Is Affected by the Annexin II2p11(2) Complex. J. Cell Biol. 1993, 123, 1119–1132.
- Rescher, U.; Gerke, V. S100A10/P11: Family, Friends and Functions. Pflug. Arch. 2007, 455, 575–582.
- Taylor, J.R.; Fernandez, D.J.; Thornton, S.M.; Skeate, J.G.; Lühen, K.P.; Da Silva, D.M.; Langen, R.; Kast, W.M. Heterotetrameric Annexin A2/S100A10 (A2t) Is Essential for Oncogenic Human Papillomavirus Trafficking and Capsid Disassembly, and Protects Virions from Lysosomal Degradation. Sci. Rep. 2018, 8, 11642.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
15 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




