Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mark Reybrouck | + 2034 word(s) | 2034 | 2021-11-29 10:03:09 | | | |
| 2 | Dean Liu | Meta information modification | 2034 | 2021-12-15 01:45:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Reybrouck, M. Neural Mechanisms of Coping with the Sounds. Encyclopedia. Available online: https://encyclopedia.pub/entry/17100 (accessed on 07 February 2026).
Reybrouck M. Neural Mechanisms of Coping with the Sounds. Encyclopedia. Available at: https://encyclopedia.pub/entry/17100. Accessed February 07, 2026.
Reybrouck, Mark. "Neural Mechanisms of Coping with the Sounds" Encyclopedia, https://encyclopedia.pub/entry/17100 (accessed February 07, 2026).
Reybrouck, M. (2021, December 14). Neural Mechanisms of Coping with the Sounds. In Encyclopedia. https://encyclopedia.pub/entry/17100
Reybrouck, Mark. "Neural Mechanisms of Coping with the Sounds." Encyclopedia. Web. 14 December, 2021.
Copy Citation
Listening to music, by definition, refers to the sensorial act of processing acoustic features by the auditory system. Hence, a description in terms of the objective acoustic characteristics may help to tackle some of the elusive aspects of possible causal relationships between music and its effects by describing at least the stimulus side of the music processing chain (input).
neuroaesthetics
musical-aesthetic experience
eudaimonic experience
1. Aesthetic Listening and the Generation of Pleasure
Neuroscience has provided major insights into the positive effects of aesthetic music listening by focusing on the localization and connectivity of so-called hedonic hotspots in cortical and subcortical regions of the brain, and by examining the role of neurotransmitters in the modulation of the physical and physiological responses to the music.
The generation of pleasure—both in its sensorial and conscious aspects— depends mainly on a network of strongly connected hedonic hotspots within the mesolimbic pathway. They have adaptive values by helping us to want, to like, and to learn about stimuli that may possibly ensure survival, and are found along the reward circuitry in the nucleus accumbens (NAcc), the insula, the orbitofrontal cortex (OFC) and the ventral pallidum [1][2]. The subcortical hedonic hotspots are responsible for the simple and spontaneous core liking reactions to pleasurable stimuli, whereas the involvement of cortical prefrontal structures is needed for the conscious feelings of wanting or incentive salience during the appetitive phase [3][4], this network shows an interplay between more evolved neocortical areas and evolutionary older areas of the brain.
Most investigations, however, have concentrated on cortical and subcortical “telencephalic sites” of aesthetic and emotional processing, such as, e.g., the dorsal and ventral striatum and amygdala, somewhat in line with a very recent meta-analysis on music listening and imagery (see Figure 1) [5]. Yet some evolutionary older levels, such as the brain stem, which houses several auditory processing mechanisms as well as core mechanisms for homeostatic regulation, have been left out to some extent, due partially to the technical difficulties of brain stem imaging, but also to the lack of theoretical frameworks for its role in hearing as related to the mechanism of homeostasis [6]. These older levels are important, however, in the sense that a distinction has been proposed between fast and slow routes of affective evaluative processes: the fast route is a primary route to the positive or negative appraisal or evaluation of stimuli, relying on quick and automatic brain responses, mostly below the level of consciousness and originating mainly in the brainstem, the primary sensory cortices, and the limbic system; the slow route, on the contrary, involves the evolutionary more evolved structures of the brain, such as the prefrontal cortex with outcomes such as the conscious liking or appraisal of a piece of art or music [7][8][9].
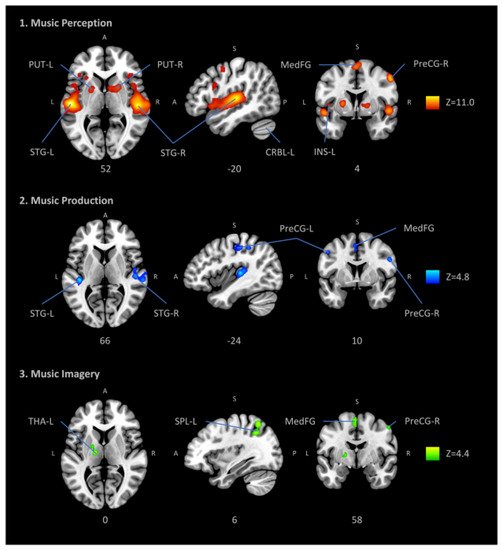
Figure 1. Automatic meta-analysis obtained with neurosynth.org of 163 studies utilizing the fMRI methodology and including “music” as keyword. Brain structures related to audition (supratemporal cortex), motor control (dorsomedial prefrontal cortex and cerebellum), body awareness (insula) and reward (ventral striatum) are visible. ROIs: PUT putamen, STG superior temporal gyrus (primary auditory cortex), MedFG medial frontal gyrus, CRBL cerebellum, INS insula, PreCG precentral gyrus (primary motor cortex or M1), THA thalamus, SPL superior parietal lobule, Z peak Z-value. (Figure reproduced without modification from [5]. (© Springer, Creative Commons Attribution))
Besides these structural issues, neurochemical research has also identified the impact of neurotransmitters on the affective aspects of music listening. The study of combined psychophysical, neurochemical, and hemodynamic effects, in particular, may reveal peaks in the autonomic nervous system activity, which explain also the effects of music on mood (e.g., [10]. Studies with ligand-based position emission tomography—using radioligand raclopride that binds with dopamine—have shown that strong emotional responses to music lead to dopamine release in the mesolimbic striatal system together with sensory regions for auditory reception while listening to highly pleasurable music [11][12].
Some findings have been even more ground-breaking, with an unforeseen functional dissociation between the “anticipatory phase” of peak emotional experiences with dopaminergic activity in the caudate nucleus, and the “consummatory phase” of the actual experience with activity in the nucleus accumbens. This dopamine release, immediately before the climax of emotional experiences, in the caudate, is of considerable importance, as this region is highly interconnected with limbic regions such as the amygdalae, hippocampus, cingulate and ventromedial prefrontal cortex, which all mediate emotional responses. The findings point also to the direction of two distinct anatomical pathways that play different but complementary roles in the emotional experience of music, mapping somewhat onto the “wanting” and “liking” dimension of music appreciation ([13], see also [14]).
Care should be taken, however, not yet to generalize too much about the role of dopamine release. Even if it underlies both the appetitive and consummatory phases of reward, the peak of the pleasure experience and the hedonic properties or subjective pleasure that is associated with obtaining a reward may depend on the release of other neurotransmitters such as endogenous opioid peptide releases in the hedonic hotspots in the nucleus accumbens, which is a region that is implicated also in the euphoric components of psychostimulants such as, e.g., cocaine [15][2][16][17][18][19] (see also [6]). This could challenge the common view that dopamine is causally related to music-evoked pleasure through the engagement of the hedonic hotspots in favor of a milder claim that it should arise from motivational signals and cognitive appraisal so as to increase the attractiveness of the surrounding environment and to strengthen the efficacy of rewarding stimuli [19]. As such, it should intervene in the processing of diverse types of pleasures, as is the case in aesthetic experiences [15][2]. Somewhat generalizing, it can thus be stated that listening to pleasurable music is associated with activation of the NAcc and its interactions with brain structures that regulate autonomic, emotional, and cognitive functions. There is, as such, a strong link between emotional and cognitive systems which link the orbitofrontal cortex with mesocorticolimbic dopaminergic circuitry (NAcc and VTA) [15].
2. Music Listening, Stress, and Allostatic Load
Music, as a candidate elicitor, does not always contribute to pleasure. As a vibrational phenomenon, it may put stress on the body and the brain, it can have a role in maintaining, restoring, or even disrupting the homeostatic balance, and can, in the worst case, even become a source of allostatic load. It makes sense, therefore, to conceive of music listening in terms of “coping behavior” (see above) with a major distinction between adaptive and maladaptive ways of listening [20][21].
The concept of adaptation, first, is very useful. According to Selye—a pioneer in the study of stress—, life is a process of adaptation to the circumstances in which we exist, and health and happiness depend on the successful adjustments to the ever-changing conditions of this environmental surrounding world [22][23]. Healthy functioning, in this view, requires ongoing adjustments and alternations of the internal physiological milieu through physiological systems that exhibit fluctuating levels of activity to respond and adapt to the solicitations of the environment [24]. Stress research, however, has traditionally focused on failures in this process, emphasizing the role of allostatic load, which, in its etymological sense, means “stability through change”, thus emphasizing the constant dynamism of our internal physiology [25]. It can be defined as the cumulative wear and tear of the strain of physiological effects of multiple forms of adversity on several organs and tissues, due to the overactive or inefficient management of the stress responses. Allostasis, then, reflects the consequences for risk of pathology, such as organ system breakdown, compromised immune response, cardiovascular dysfunction and disease, elevated cortisol and insulin secretion, accumulation of abdominal fat, loss of bone minerals, reproductive impairments, decreased neurogenesis, increased neuronal cell death and associated atrophy in the limbic system ([26], see also [27]).
Two major kinds of allostatic load have been identified thus far: “physiological reactivity”, as in the case of acute shifts and elevations in physiological activity in response to threatening stimuli, and “chronic elevations” beyond the basal operating ranges, operating mainly in the absence of challenging stimuli. Conditions as hypertension and diabetes are typical examples. The picture, however, is not exclusively negative as it is also possible to conceive of optimal allostasis. It is a challenging approach that has marked a kind of paradigm shift in stress research by focusing not exclusively on failures in the adaptation process [24]. An operational approach to such optimal allostasis, therefore, should include not only the maintenance of load indicators in normal operating ranges but also the measurement of selected brain opioids such as β-Endorphins and leucine and methionine enkephalins, which have powerful effects in counteracting negative emotions and favoring positive ones [28][29]. The release of dopamine from the catecholamine systems, the central nervous system opioid peptides, and oxytocin are also of particular importance in this regard (see [24] for an overview).
The effects of allostatic load, further, are reflected in biological stress responses, such as the neuroendocrine, autonomic, and immune system responses, which put high strains on the mobilization from the hypothalamic-pituitary-adrenal axis [15], with a corresponding cascade of hormone secretions, going from the hypothalamic-driven release of corticotropin-releasing factor (CRF), to the pituitary-driven release of corticotropin to the adrenal-driven release of cortisol [24][30]. Activation of the sympathetic nervous system by stress-inducing stimuli, further, results in the release of catecholamines such as norepinephrine and epinephrine, driven by the medulla of the adrenal gland. The immune function, finally, can be compromised by the workings of these hormones and transmitters.
The above-described neurotransmitter-mediated neural activity within the reward pathways, on the other hand, regulates emotions and mood through changes in autonomic and physiological responses during music listening. This often leads to a relaxed smoothened state through psychological processes of decentering and non-attachment, so as to decouple the “sensory” and “affective” components of stressors with a resulting reduction of the sympathetic tone and suppression of mobilization by the hypothalamic-pituitary-adrenal axis. The aim of such decentering is to decrease stress responses to innocuous cues and to foster a rapid return to physiological and emotional baseline situations in response to real threats. Such reductions should then be visible across physiological mediators such as the adrenomedullary catecholamines (epinephrine and norepinephrine), adrenocortical glucocorticoids (cortisol), pituitary hormones (ACTH, prolactin, and growth hormones), and cytokines from cells of the immune system (IL-1, IL-6, and TNF-α) [31].
Hence, music can have a mediating role in the interactions between these physiological organ systems with a possible regulation of systemic stress hormone levels. Two markers of the HPA axis—ß-endorphin and cortisol—, have been found to decrease as the result of engaging with music, but not all findings point in the direction of lowering levels of activation. Stimulating music, e.g., can induce increased levels of plasma cortisol, ACTH, prolactin, growth hormone, and norepinephrine [32].
Generalizing a little, it can be stated that music can regulate stress, arousal, and emotions by initiating reflexive brainstem-mediated responses, which include heart rate, blood pressure, skin conductance, and muscle tension [33]. A distinction should be made, however, between those levels of stress that are perceived as harmful or annoying and those that are experienced as being beneficial for better coping behavior. It is a conception that is related to the psychobiological model of arousal, which states that enjoyment is optimal at intermediate arousal levels.
Applied to music, this means that it makes sense to attune ourselves to sound environments and sonic landscapes, including music, that provides stimulation in the optimal arousal zone [20][34][35], allowing us to cultivate positive adaptive reactions to beneficial stressors as well as to avoid possible distress triggered by harmful stimuli. It brings us to Selye’s concept of eustress, which he contrasted with distress, as the syndrome that is triggered by unspecific harmful stimuli or activities [22][23][36]. Eustress represents the pleasant stress of fulfillment, including both the properties of the stressor, considered to be beneficial in that case, the effort that is valued in terms of positive valence, and the effects that guarantee no damaging outcomes. Stressors, then, can be considered beneficial when they do not exceed the capacity for the maintenance or restoration of homeostasis [37].
Both negative-aversive and positive-rewarding stress, however, is associated with increased activation of the HPA axis. It means, finally, that aesthetic engagement with music reveals a unique neural architecture that connects physiological responses typical of stress to the combination of positive and negative affect, which leads ultimately to an expansion of our cognitive-emotional states. This is exemplified most typically in the experience of awe and aesthetic chills, which are characterized by vastness and the need for accommodation [38][39].
References
- Lindquist, K.; Wager, T.; Kober, H.; Bliss-Moreau, E.; Feldman Barrett, L. The brain basis of emotion: E meta-analytic review. Behav. Brain Sci. 2012, 35, 121–143.
- Peciña, S.; Smith, K.; Berridge, K. Hedonic hot spots in the brain. Neuroscientist 2006, 12, 500–511.
- Belfi, A.; Loui, P. Musical anhedonia and rewards of music listening: Current advances and a proposed model. Ann. N. Y. Acad. Sci. 2020, 1464, 99–114.
- Ferreri, L.; Mas-Herrero, E.; Zatorre, R.; Ripollés, P.; Gomez-Andresa, A.; Alicarta, H.; Olivé, G.; Marco-Pallarés, J.; Antonijoan, R.; Vallei, M.; et al. Dopamine modulates the reward experiences elicited by music. Proc. Natl. Acad. Sci. USA 2019, 116, 3793–3798.
- Kringelbach, M.L. The Pleasure Center. Trust your Animal Instincts; Oxford University Press: Oxford, UK, 2009.
- Liu, C.; Brattico, E.; Abu-Jamous, B.; Pereira, C.; Jacobsen, T.; Nandi, A. Effect of Explicit Evaluation on Neural Connectivity Related to Listening to Unfamiliar Music. Front. Hum. Neurosci. 2017, 11, 611.
- Pando-Naude, V.; Patyczek, A.; Bonetti, L.; Vuust, P. An ALE meta-analytic review of top-down and bottom-up processing of music in the brain. Sci. Rep. 2020, 11, 20813.
- Jacobsen, T.; Schubotz, R.I.; Hofel, L.; Cramon, D.Y. Brain correlates of aesthetic judgment of beauty. Neuroimage 2006, 276–285.
- LeDoux, J.E. Emotion, memory and the brain. Sci. Am. 1994, 270, 50–57.
- MacDonald, R.; Kreutz, G.; Mitchell, L. (Eds.) Music Health and Wellbeing; Oxford University Press: Oxford, UK, 2012.
- Nadal, M.; Skov, M. Introduction to the Special Issue: Toward an Interdisciplinary Neuroaesthetics. Psychol. Aesthet. Creat. Arts 2013, 7, 1–12.
- Zatorre, R.J.; Salimpoor, V.N. From perception to pleasure: Music and its neural substrates. Proc. Natl. Acad. Sci. USA 2013, 110, 10430–10437.
- Power, M.; Dalgleish, T. Two routes to emotion: Some implications of multilevel theories of emotion for therapeutic practice. Behav. Cogn. Psychother. 1999, 27, 129–141.
- Waterman, A. Two Conceptions of Happiness: Contrasts of Personal Expressiveness (Eudaimonia) and Hedonic Enjoyment. J. Pers. Soc. Psychol. 1993, 64, 678–691.
- Persico, G.; Antolini, L.; Vergani, P.; Costantini, W.; Nardi, M.T.; Bellotti, L. Maternal singing of lullabies during pregnancy and after birth: Effects on mother–infant bonding and on newborns’ behaviour. Concurrent Cohort Study. Women Birth 2017, 30, e214–e220.
- Salimpoor, V.N.; Benovoy, M.; Larcher, K.; Dagher, A.; Zatorre, R. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat. Neurosci. 2011, 14, 257–262.
- Berridge, K.C. Motivation concepts in behavioral neuroscience. Physiol. Behav. 2004, 81, 179–209.
- Berridge, K.C. The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology 2007, 191, 391–431.
- Berridge, K.; Kringelbach, M. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology 2008, 199, 457–480.
- Reybrouck, M.; Podlipniak, P.; Welch, D. Music Listening as Coping Behavior: From Reactive Response to Sense-Making. Behav. Sci. 2020, 10, 119.
- Wise, R. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004, 5, 483–494.
- Miranda, D.; Claes, M. Music listening, coping, peer affiliation and depression in adolescence. Psychol. Music 2009, 37, 215–233.
- Selye, H. The Stress of Life; McGraw-Hill: New York, NY, USA, 1956.
- Selye, H. Stress without Distress; Springer Science and Business Media LLC: Berlin, Germany, 1976.
- Ryff, C.; Singer, B. The Contours of Positive Health. Psychol. Inq. 1988, 9, 1–28.
- Sterling, P.; Eyer, J. Allostasis: A new paradigm to explain arousal pathology. In Handbook of Life Stress Cognition and Health; Fisher, J., Reason, J., Eds.; Wiley: New York, NY, USA, 1988; pp. 629–649.
- McEwen, B.S. Stress, adaptation, and disease. Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44.
- Vago, D.; Silbersweig, D. Self-awareness, self-regulation, and self-transcendence(S-ART): A framework for understanding the neurobiological mechanisms of mindfulness. Front. Hum. Neurosci. 2012, 6, 296.
- Panksepp, J. Brain opioids-A neurochemical substrate for narcotic and social dependence. In Theory in Psychopharmacology; Cooper, S., Ed.; Academic: New York, NY, USA, 1981; pp. 149–175.
- Panksepp, J. Neurochemical control of moods and emotions: Amino acids to neuropeptides. In Handbook of Emotions; Lewis, M., Haviland, J.M., Eds.; Guilford: New York, NY, USA, 1993; pp. 87–106.
- Sapolsky, R.M. Stress in the wild. Sci. Am. 1990, 262, 116.
- Gerra, G.; Zaimovic, A.; Franchini, D.; Palladino, M.; Giucastro, G.; Reali, N.; Maestri, D.; Caccavari, R.; Delsignore, R.; Brambilla, F. Neuroendocrine responses of healthy volunteers to ‘techno-music’: Relationships with personality traits and emotional state. Int. J. Psychophysiol. 1998, 28, 99–111.
- McEwen, B. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008, 583, 174–185.
- Chapados, C.; Levitin, D.J. Cross-modal interactions in the experience of musical performances: Physiological correlates. Cognition 2008, 108, 639–651.
- Saarikallio, S.; Erkkilä, J. The role of music in adolescents’ mood regulation. Psychol. Music 2007, 35, 88–109.
- Van den Tol, A.; Edwards, J.; Heflick, N. Sad music as a means for acceptance-based coping. Mus. Sci. 2016, 20, 68–83.
- Selye, H. The stress syndrome. AJN 1965, 65, 97–99.
- Perrez, M. Eustress. In The Oxford Companian to Emotion and the Affective Sciences; Sander, D., Scherer, K., Eds.; Oxford University Press: Oxford, NY, USA, 2009; p. 158.
- Keltner, D.; Haidt, J. Approaching awe, a moral, spiritual, and aesthetic emotion. Cognit. Emot. 2003, 17, 297–314.
More
Information
Subjects:
Music
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
15 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




