
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | benedetta piccardi | + 1866 word(s) | 1866 | 2021-11-30 03:22:28 | | | |
| 2 | Camila Xu | Meta information modification | 1866 | 2021-12-15 02:17:15 | | | | |
| 3 | Lindsay Dong | Meta information modification | 1866 | 2022-03-28 05:01:49 | | |
Video Upload Options
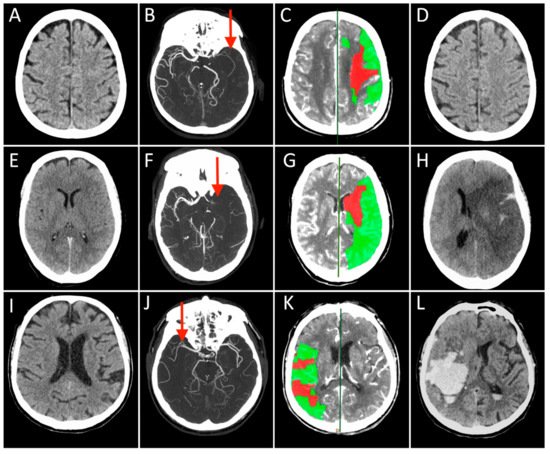
The approach to reperfusion therapies in stroke patients is rapidly evolving, but there is still no explanation why a substantial proportion of patients have a poor clinical prognosis despite successful flow restoration. This issue of futile recanalization is explained here by three clinical cases which, despite complete recanalization, have very different outcomes. Preclinical research is particularly suited to characterize the highly dynamic changes in acute ischemic stroke and to identify potential treatment targets useful for clinical translation. This entry surveys the efforts taken so far to achieve mouse models capable to investigate of investigating the neurovascular underpinnings of futile recanalization. We highlight the translational potential of targeting tissue reperfusion in fully recanalized mouse models and of investigating the underlying pathophysiological mechanisms from subcellular to tissue scale. We suggest that stroke preclinical research should increasingly drive forward a continuous and circular dialogue with clinical research. When the preclinical and the clinical stroke research are consistent, translational success will follow.
1. Introduction
2. From Bedside
References
- Nie, X.; Pu, Y.; Zhang, Z.; Liu, X.; Duan, W.; Liu, L. Futile Recanalization after Endovascular Therapy in Acute Ischemic Stroke. Biomed. Res. Int. 2018, 2018, 5879548.
- Stoll, G.; Pham, M. Beyond recanalization—A call for action in acute stroke. Nat. Rev. Neurol. 2020, 16, 591–592.
- Ter Schiphorst, A.; Charron, S.; Hassen, W.B.; Provost, C.; Naggara, O.; Benzakoun, J.; Seners, P.; Turc, G.; Baron, J.C.; Oppenheim, C. Tissue no-reflow despite full recanalization following thrombectomy for anterior circulation stroke with proximal occlusion: A clinical study. J. Cereb. Blood Flow Metab. 2021, 41, 253–266.
- Hacke, W.; Schwab, S.; Horn, M.; Spranger, M.; De Georgia, M.; von Kummer, R. ‘Malignant’ middle cerebral artery territory infarction: Clinical course and prognostic signs. Arch. Neurol. 1996, 53, 309–315.
- Brogan, M.E.; Manno, E.M. Treatment of malignant brain edema and increased intracranial pressure after stroke. Curr. Treat. Opt. Neurol. 2015, 17, 327.
- Shaw, C.M.; Alvord, E.C., Jr.; Berry, R.G. Swelling of the brain following ischemic infarction with arterial occlusion. Arch. Neurol. 1959, 1, 161–177.
- Juttler, E.; Schellinger, P.D.; Aschoff, A.; Zweckberger, K.; Unterberg, A.; Hacke, W. Clinical review: Therapy for refractory intracranial hypertension in ischaemic stroke. Crit. Care 2007, 11, 231.
- Cook, A.M.; Morgan Jones, G.; Hawryluk, G.W.J.; Mailloux, P.; McLaughlin, D.; Papangelou, A.; Samuel, S.; Tokumaru, S.; Venkatasubramanian, C.; Zacko, C.; et al. Guidelines for the Acute Treatment of Cerebral Edema in Neurocritical Care Patients. Neurocrit. Care 2020, 32, 647–666.
- Battey, T.W.; Karki, M.; Singhal, A.B.; Wu, O.; Sadaghiani, S.; Campbell, B.C.; Davis, S.M.; Donnan, G.A.; Sheth, K.N.; Kimberly, W.T. Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke 2014, 45, 3643–3648.
- Del Zoppo, G.J. The neurovascular unit in the setting of stroke. J. Intern. Med. 2010, 267, 156–171.
- Stokum, J.A.; Gerzanich, V.; Simard, J.M. Molecular pathophysiology of cerebral edema. J. Cereb. Blood Flow Metab. 2016, 36, 513–538.
- Bernier, L.P.; Brunner, C.; Cottarelli, A.; Balbi, M. Location Matters: Navigating Regional Heterogeneity of the Neurovascular Unit. Front. Cell Neurosci. 2021, 15, 696540.
- Schaeffer, S.; Iadecola, C. Revisiting the neurovascular unit. Nat. Neurosci. 2021, 24, 1198–1209.
- Yang, G.Y. Advancement in stroke research. Stroke Vasc. Neurol. 2019, 4, 61–62.
- Zhou, M.; Shi, S.X.; Liu, N.; Jiang, Y.; Karim, M.S.; Vodovoz, S.J.; Wang, X.; Zhang, B.; Dumont, A.S. Caveolae-Mediated Endothelial Transcytosis across the Blood-Brain Barrier in Acute Ischemic Stroke. J. Clin. Med. 2021, 10, 3795.
- Dong, M.X.; Hu, Q.C.; Shen, P.; Pan, J.X.; Wei, Y.D.; Liu, Y.Y.; Ren, Y.F.; Liang, Z.H.; Wang, H.Y.; Zhao, L.B.; et al. Recombinant Tissue Plasminogen Activator Induces Neurological Side Effects Independent on Thrombolysis in Mechanical Animal Models of Focal Cerebral Infarction: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158848.
- Hussein, H.M.; Saleem, M.A.; Qureshi, A.I. Rates and predictors of futile recanalization in patients undergoing endovascular treatment in a multicenter clinical trial. Neuroradiology 2018, 60, 557–563.
- Lee, S.H.; Kim, B.J.; Han, M.K.; Park, T.H.; Lee, K.B.; Lee, B.C.; Yu, K.H.; Oh, M.S.; Cha, J.K.; Kim, D.H.; et al. Futile reperfusion and predicted therapeutic benefits after successful endovascular treatment according to initial stroke severity. BMC Neurol. 2019, 19, 11.
- Alawieh, A.; Vargas, J.; Fargen, K.M.; Langley, E.F.; Starke, R.M.; De Leacy, R.; Chatterjee, R.; Rai, A.; Dumont, T.; Kan, P.; et al. Impact of Procedure Time on Outcomes of Thrombectomy for Stroke. J. Am. Coll. Cardiol. 2019, 73, 879–890.
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Davalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731.
- Leiva-Salinas, C.; Jiang, B.; Wintermark, M. Computed Tomography, Computed Tomography Angiography, and Perfusion Computed Tomography Evaluation of Acute Ischemic Stroke. Neuroimaging Clin. N. Am. 2018, 28, 565–572.
- Menon, B.K.; d’Esterre, C.D.; Qazi, E.M.; Almekhlafi, M.; Hahn, L.; Demchuk, A.M.; Goyal, M. Multiphase CT Angiography: A New Tool for the Imaging Triage of Patients with Acute Ischemic Stroke. Radiology 2015, 275, 510–520.
- Wintermark, M.; Flanders, A.E.; Velthuis, B.; Meuli, R.; van Leeuwen, M.; Goldsher, D.; Pineda, C.; Serena, J.; van der Schaaf, I.; Waaijer, A.; et al. Perfusion-CT assessment of infarct core and penumbra: Receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 2006, 37, 979–985.
- Casetta, I.; Fainardi, E.; Saia, V.; Pracucci, G.; Padroni, M.; Renieri, L.; Nencini, P.; Inzitari, D.; Morosetti, D.; Sallustio, F.; et al. Endovascular Thrombectomy for Acute Ischemic Stroke Beyond 6 Hours from Onset: A Real-World Experience. Stroke 2020, 51, 2051–2057.
- Muddasani, V.; de Havenon, A.; McNally, J.S.; Baradaran, H.; Alexander, M.D. MR Perfusion in the Evaluation of Mechanical Thrombectomy Candidacy. Top. Magn. Reson. Imaging 2021, 30, 197–204.
- Kamalian, S.; Lev, M.H. Stroke Imaging. Radiol. Clin. N. Am. 2019, 57, 717–732.
- Atchaneeyasakul, K.; Shang, T.; Haussen, D.; Ortiz, G.; Yavagal, D. Impact of MRI Selection on Triage of Endovascular Therapy in Acute Ischemic Stroke: The MRI in Acute Management of Ischemic Stroke (MIAMIS) Registry. Interv. Neurol. 2020, 8, 135–143.
- Zang, N.; Lin, Z.; Huang, K.; Pan, Y.; Wu, Y.; Wu, Y.; Wang, S.; Wang, D.; Ji, Z.; Pan, S. Biomarkers of Unfavorable Outcome in Acute Ischemic Stroke Patients with Successful Recanalization by Endovascular Thrombectomy. Cerebrovasc. Dis. 2020, 49, 583–592.
- Makris, K.; Haliassos, A.; Chondrogianni, M.; Tsivgoulis, G. Blood biomarkers in ischemic stroke: Potential role and challenges in clinical practice and research. Crit. Rev. Clin. Lab. Sci. 2018, 55, 294–328.




